Abstract
Background/Objectives:
Consumption of whole-grain products is known to have beneficial effects on human health. The effects of whole-grain products on the intestinal microbiota and intestinal integrity have, however, only been studied limitedly. We investigate changes of the human gut microbiota composition after consumption of whole-grain (WW) or refined wheat (RW) and further study effects on gut wall integrity.
Subjects/Methods:
Quantitative PCR was used to determine changes in the gut bacterial composition in postmenopausal women following a 12-week energy-restricted dietary intervention with WW (N=38) or RW (N=34). Intestinal integrity was determined by measuring trans-epithelial resistance (TER) across a Caco-2 cell monolayer, following exposure to faecal water.
Results:
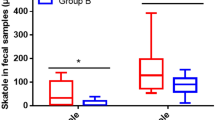
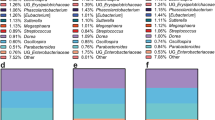
No significant differences in microbiota composition were observed between the two dietary groups; however, the whole-grain intervention increased the relative abundance of Bifidobacterium compared to baseline, supporting a prebiotic effect of whole-grain wheat. Faecal water increased TER independent of dietary intervention, indicating that commensal bacteria produce metabolites that generally provide a positive effect on intestinal integrity. Combining microbiota composition data from the run-in period with its effect on TER revealed a tendency for a negative correlation between the relative abundance of Bifidobacterium and TER (P=0.09). This contradicts previous findings but supports observations of increased Salmonella infection in animal models following treatment with bifidogenic prebiotics.
Conclusions:
The present study shows that whole-grain wheat consumption increases the abundance of bifidobacteria compared to baseline and may have indirect effects on the integrity of the intestinal wall.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jonnalagadda SS, Harnack L, Liu RH, McKeown N, Seal C, Liu S et al. Putting the whole grain puzzle together: health benefits associated with whole grains - summary of American Society for Nutrition 2010 Satellite Symposium. J Nutr 2011; 141: 1011S–1022S.
Okarter N, Liu RH . Health benefits of whole grain phytochemicals. Crit Rev Food Sci Nutr 2010; 50: 193–208.
Gibson GR, Roberfroid MB . Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995; 125: 1401–1412.
Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 2008; 295: G1025–G1034.
Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr 2008; 99: 110–120.
Carvalho-Wells AL, Helmolz K, Nodet C, Molzer C, Leonard C, McKevith B et al. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: a human feeding study. Br J Nutr 2010; 104: 1353–1356.
Lappi J, Salojarvi J, Kolehmainen M, Mykkanen H, Poutanen K, de Vos WM et al. Intake of whole-grain and fiber-rich rye bread versus refined wheat bread does not differentiate intestinal microbiota composition in Finnish adults with metabolic syndrome. J Nutr 2013; 143: 648–655.
Ross AB, Bruce SJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Bourgeois A et al. A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. Br J Nutr 2011; 105: 1492–1502.
Connolly ML, Lovegrove JA, Tuohy KM . In vitro evaluation of the microbiota modulation abilities of different sized whole oat grain flakes. Anaerobe 2010; 16: 483–488.
Connolly ML, Tuohy KM, Lovegrove JA . Wholegrain oat-based cereals have prebiotic potential and low glycaemic index. Br J Nutr 2012; 108: 2198–2206.
Connolly ML, Lovegrove JA, Tuohy KM . In vitro fermentation characteristics of whole grain wheat flakes and the effect of toasting on prebiotic potential. J Med Food 2012; 15: 33–43.
Maccaferri S, Klinder A, Cacciatore S, Chitarrari R, Honda H, Luchinat C et al. In vitro fermentation of potential prebiotic flours from natural sources: Impact on the human colonic microbiota and metabolome. Mol Nutr Food Res 2012; 56: 1342–1352.
Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A et al. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J 2013; 7: 1933–1943.
Shen L, Weber CR, Raleigh DR, Yu D, Turner JR . Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 2011; 73: 283–309.
Bovee-Oudenhoven IM, Ten Bruggencate SJ, Lettink-Wissink ML, van der Meer R . Dietary fructo-oligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of salmonella in rats. Gut 2003; 52: 1572–1578.
Petersen A, Bergström A, Andersen JB, Hansen M, Lahtinen SJ, Wilcks A et al. Analysis of the intestinal microbiota of oligosaccharide fed mice exhibiting reduced resistance to Salmonella infection. Benef Microbes 2010; 1: 271–281.
Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, van der Meer R . Dietary fructo-oligosaccharides dose-dependently increase translocation of salmonella in rats. J Nutr 2003; 133: 2313–2318.
Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, van der Meer R . Dietary fructooligosaccharides increase intestinal permeability in rats. J Nutr 2005; 135: 837–842.
Raschka L, Daniel H . Mechanisms underlying the effects of inulin-type fructans on calcium absorption in the large intestine of rats. Bone 2005; 37: 728–735.
Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 2012; 142: 710–716.
Bergström A, Licht TR, Wilcks A, Andersen JB, Schmidt LR, Gronlund HA et al. Introducing GUt low-density array (GULDA): a validated approach for qPCR-based intestinal microbial community analysis. FEMS Microbiol Lett 2012; 337: 38–47.
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL . Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012; 13: 134.
Ramakers C, Ruijter JM, Deprez RH, Moorman AF . Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 2003; 339: 62–66.
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 2009; 37: e45.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI . Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005; 102: 11070–11075.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI . Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023.
Ouwehand AC, Bergsma N, Parhiala R, Lahtinen S, Gueimonde M, Finne-Soveri H et al. Bifidobacterium microbiota and parameters of immune function in elderly subjects. FEMS Immunol Med Microbiol 2008; 53: 18–25.
Junick J, Blaut M . Quantification of human fecal Bifidobacterium species by use of quantitative real-time PCR analysis targeting the groEL gene. Appl Environ Microbiol 2012; 78: 2613–2622.
Gill CI, Heavey P, McConville E, Bradbury I, Fassler C, Mueller S et al. Effect of fecal water on an in vitro model of colonic mucosal barrier function. Nutr Cancer 2007; 57: 59–65.
Commane DM, Shortt CT, Silvi S, Cresci A, Hughes RM, Rowland IR . Effects of fermentation products of pro- and prebiotics on trans-epithelial electrical resistance in an in vitro model of the colon. Nutr Cancer 2005; 51: 102–109.
Lopez P, Gonzalez-Rodriguez I, Sanchez B, Ruas-Madiedo P, Suarez A, Margolles A et al. Interaction of Bifidobacterium bifidum LMG13195 with HT29 cells influences regulatory-T-cell-associated chemokine receptor expression. Appl Environ Microbiol 2012; 78: 2850–2857.
Cani PD, Possemiers S, Van de WT, Guiot Y, Everard A, Rottier O et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009; 58: 1091–1103.
Petersen A, Heegaard PM, Pedersen AL, Andersen JB, Sørensen RB, Frokiaer H et al. Some putative prebiotics increase the severity of Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol 2009; 9: 245.
Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, Katan MB, van der Meer R . Dietary fructo-oligosaccharides and inulin decrease resistance of rats to salmonella: protective role of calcium. Gut 2004; 53: 530–535.
Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, Katan MB, van der Meer R . Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J Nutr 2006; 136: 70–74.
Walter J, Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Loach DM, Munro K et al. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl Environ Microbiol 2000; 66: 297–303.
Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K . Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 2008; 47: 367–373.
Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP . Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 2001; 67: 2578–2585.
Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM . Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol 2002; 68: 114–123.
Bartosch S, Fite A, Macfarlane GT, McMurdo ME . Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 2004; 70: 3575–3581.
Delroisse JM, Boulvin AL, Parmentier I, Dauphin RD, Vandenbol M, Portetelle D . Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res 2008; 163: 663–670.
Matsuki T, Watanabe K, Tanaka R, Oyaizu H . Rapid identification of human intestinal bifidobacteria by 16S rRNA-targeted species- and group-specific primers. FEMS Microbiol Lett 1998; 167: 113–121.
Acknowledgements
The project was funded by the European Commission in the Communities 6th Framework Programme, Project HEALTHGRAIN (FOOD-CT-2005-514008) and by the Gut, Grain & Greens Research Centre, supported by the Danish Strategic Research Council (11-116163). We thank Bodil Madsen and Kate Vina Vibefeldt for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Christensen, E., Licht, T., Kristensen, M. et al. Bifidogenic effect of whole-grain wheat during a 12-week energy-restricted dietary intervention in postmenopausal women. Eur J Clin Nutr 67, 1316–1321 (2013). https://doi.org/10.1038/ejcn.2013.207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.207
Keywords
This article is cited by
-
Dietary approaches to stop hypertension (DASH) diet improves hepatic fibrosis, steatosis and liver enzymes in patients with non-alcoholic fatty liver disease: a randomized controlled trial
European Journal of Nutrition (2024)
-
Dietary Xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats
BMC Research Notes (2014)