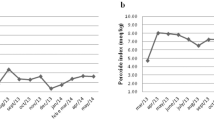
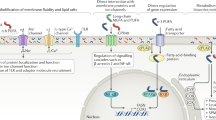
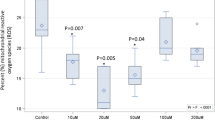
Abstract
An increase in adiposity is associated with altered levels of biologically active proteins. These include the hormones adiponectin and leptin. The marked change in circulating concentrations of these hormones in obesity has been associated with the development of insulin resistance and metabolic syndrome. Variations in dietary lipid consumption have also been shown to impact obesity. Specifically, omega-3 fatty acids have been correlated with the prevention of obesity and subsequent development of chronic disease sequalae. This review explores animal and human data relating to the effects of omega-3 fatty acids (marine lipids) on adiponectin and leptin, considering plausible mechanisms and potential implications for obesity management. Current evidence suggests a positive, dose-dependent relationship between omega-3 fatty acid intake and circulating levels of adiponectin. In obese subjects, this may translate into a reduced risk of developing cardiovascular disease, metabolic syndrome and diabetes. In non-obese subjects, omega-3 is observed to decrease circulating levels of leptin; however, omega-3-associated increases in leptin levels have been observed in obese subjects. This may pose benefits in the prevention of weight regain in these subjects following calorie restriction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K . Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006; 116: 1784–1792.
Zhang F, Basinski M, Beals J, Briggs S, Churgay L, Clawson D et al. Human obesity protein, leptin. RCSB Protein Data Bank. Nature 1997; 387: 206–209.
Krauss R, Eckel R, Howard B, Appel L, Daniels S, Deckelbaum R et al. AHA Dietary Guidelines Revision 2000: a statement for healthcare professionals from the nutrition committee of the American Heart Association. Circulation 2000; 102: 2284–2299.
Meier U, Gressner A . Endocrine Regulation of Energy Metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin and resistin. Clin Chem 2004; 50: 1511–1525.
Knudson J, Dick G, Tune J . Adipokines and coronary vasomotor dysfunction. Exp Biol Med 2007; 232: 727–736.
Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J 2006; 27: 2300–2309.
Kaidar-Person O, Person B, Szomstein S, Rosenthal R . Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: Vitamins. Obes Surg 2008; 18: 870–876.
Ren J . Leptin and hyperleptinemia—from friend to foe for cardiovascular function. J Endocrinol 2004; 181: 1–10.
Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace A et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation 2006; 114: 623–629.
Civitarese A, Ukropcova B, Carling S, Hulver M, DeFronzo R, Mandarino L et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 2006; 4: 75–87.
Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002; 8: 731–737.
Dekker J, Funahashi T, Nijpels G, Pilz S, Stehouwer C, Snijder M et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab 2008; 93: 1489–1496.
Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart 2004; 90: 528–533.
Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB . Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004; 291: 1730–1737.
Considine R, Sinha V, Heiman M, Kriauciunas A, Stephens T, Nyce M et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334: 292–295.
Frederich R, Hamann A, Anderson S, Lollmann V, Lowell B, Flier J . Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1995; 1: 1311–1314.
Skurk T, Alberti-Huber C, Herder C, Hauner H . Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007; 92: 1023–1033.
Houseknecht K, Baile C, Matteri R, Spurlock M . The biology of leptin: a review. J Anim Sci 1998; 76: 1405–1420.
Auwerx J, Staels B . Leptin. Lancet 1998; 351: 737–742.
Jequier E . Leptin signaling, adiposity, and energy balance. Ann NY Acad Sci 2002; 967: 379–388.
Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL . Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 1997; 82: 561–565.
Cameron AJ, Welborn TA, Zimmet PZ, Dunstan DW, Owen N, Salmon J et al. Overweight and obesity in Australia: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). MJA 2003; 178: 427–432.
Madsen E, Bruun J, Skogstrand K, Hougaard D, Christiansen T, Richelsen B . Long-term weight loss decreases the nontraditional cardiovascular risk factors interleukin-18 and matrix metalloproteinase-9 in obese subjects. Metabolism 2009; 58: 946–953.
Jequier E . Obesity. Impairment of energy intake or of energy expenditure. Ann Endocrinol (Paris) 1995; 56: 87–92.
Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM . Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 1997; 138: 2569–2576.
Friedman J, Halass J . Leptin and the regulation of body weight in mammals. Nature 1998; 395: 763–777.
Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ et al. Leptin regulates proinflammatory immune responses. FASEB J 1998; 12: 57–65.
Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation 2004; 109: 2181–2185.
Corsonello A, Perticone F, Malara A, De Domenico D, Loddo S, Buemi M et al. Leptin-dependent platelet aggregation in healthy, overweight and obese subjects. Int J Obes Relat Metab Disord 2003; 27: 566–573.
Konstantinides S, Schafer K, Loskutoff DJ . The prothrombotic effects of leptin possible implications for the risk of cardiovascular disease in obesity. Ann NY Acad Sci 2001; 947: 134–141. discussion 41–2.
Rolls B, Drewnowski A, Ledikwe J . Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc 2005; 105: S98–S103.
Moussavi N, Gavino V, Receveur O . Could the quality of dietary fat, and not just its quantity, be related to risk of obesity? Obesity 2008; 16: 7–15.
Duncan K, Bacon I, Weinsier R . The effects of high and low energy density diets on satiety, energy intake, and eating time of obese and nonobese subjects. Am J Clin Nutr 1983; 37: 763–767.
Johansson L, Solvoll K, Bjorneboe G, Drevon C . Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr 1998; 68: 266–274.
Micallef M, Munro I, Phang M, Garg M . Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Brit J Nutr 2009; 102: 1370–1374.
Flachs P, Mohamed-Ali V, Horakova O, Rossmeisl M, Hosseinzadeh-Attar M, Hensler M et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006; 49: 394–397.
Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Am Heart Assoc J 2007; 27: 1918–1925.
Marchioli R, Valagussa F . The results of the GISSI-Prevenzione trial in the general framework of secondary prevention. Eur Heart J 2000; 21: 949–952.
Ruxton C, Reed S, Simpson M, Millington K . The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 2004; 17: 449–459.
Mozaffarian D, Rimm EB . Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006; 296: 1885–1899.
Wang Z, Al-Regaiey KA, Masternak MM, Bartke A . Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Ferontol Ser A Biol Sci Med Sci 2006; 61A: 323–331.
Nahin R, Barnes P, Stussman B, Bloom B . Costs of Complementary and Alternative Medicine (CAM) and Frequency of Visits to CAM Practitioners: United States, 2007. US Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, USA, 2009.
National Institutes of Health. Get the Facts: Omega-3 Supplements: An Introduction. Department of Health and Human Services: Bethesda, MD, USA, 2012.
Mori T, Bao D, Burke V, Puddey I, Watts G, Beilin L . Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr 1999; 70: 817–825.
Simopoulos A . Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999; 70 (suppl), S560–S569.
Simopoulos A . Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 2002; 21: 495–505.
Serhan C, Arita M, Hong S, Gotlinger K . Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirintriggered epimers. Lipids 2004; 39: 1125–1132.
Babcock T, Kurland A, Helton W, Rahman A, Anwar K, Espat N . Inhibition of activator protein-1 transcription factor activation by omega-3 fatty acid modulation of mitogen-activated protein kinase signaling kinases. Parenter Enteral Nutr 2003; 27: 176–180.
Ren B, Thelen A, Peters J, Gonzalez F, Jump D . Polyunsaturated fatty acid suppression of hepatic fatty acid. Synthase and S14 gene expression does not require peroxisome proliferator-activated receptor alpha. J Biol Chem 1997; 272: 26827–26832.
Harris W, Miller M, Tighe A, Davidson M, Schaefer E . Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 2008; 197: 12–24.
Morris M, Sacks F, Rosnerm B . Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993; 88: 523–533.
Appel L, Miller E, Seidler A, Whelton P . Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med 1993; 153: 1429–1438.
Harris W . n-3 Fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997; 65: 1645–54S.
Hansson G . Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685–1695.
Hotamisligil G, Arner P, Caro J, Atkinson R, Spiegelman B . Increased adipose tissue expression of tumor necrosis factor-a in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409–2415.
Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R et al. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis 2009; 202: 263–271.
Heart Foundation Australia. Fish, fish oils, n-3 polyunsaturated fatty acids and cardiovascular health. Heart Foundation Australia 2008, [cited 25 May 2010]; Available from http://www.heartfoundation.org.au/SiteCollectionDocuments/HW_FS_FishOils_PS_FINAL_web.pdf.
Rizos E, Ntzani E, Bika E, Kostapanos M, Elisaf M . Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012; 308: 1024–1033.
Holub B . Clinical nutrition: omega-3 fatty acids in cardiovascular care. CMAJ 2002; 166: 608–615.
Jorgensen M, Bjeregaard P, Borch- Johnsen K . Diabetes and impaired glucose tolerance among the Inuit population of Greenland. Diabetes Care 2002; 25: 1766–1771.
Mouratoff G, Scott E . Diabetes mellitus in Eskimos after a decade. JAMA 1973; 226: 1345–1346.
Young T, Schraer C, Shubnikoff E, Szathmary E, Nikitin Y . Prevalence of diagnosed diabetes in circumpolar indigenous populations. Int J Epidemiol 1992; 21: 730–736.
Burrows N, Geiss L, Engelgau M, Acton K . Prevalence of diabetes among Native Americans and Alaska Natives, 1990–1997: an increasing burden. Diabetes Care 2000; 23: 1786–1790.
Simonsen T, Vartun A, Lyngmo V, Nordoy A . Coronary heart disease, serum lipids, platelets and dietary fish in two communities in northern Norway. Acta Med Scand 1987; 222: 237–245.
Adler A, Boyko E, Schraer C, Murphy N . Lower prevalence of impaired glucose tolerance and diabetes associated with daily seal oil or salmon consumption among Alaska Natives. Diab Care 1994; 17: 1498–1501.
Feskens E, Virtanen S, Rasanen L, Tuomilehto J, Stengard J, Pekkanen J et al. Dietary factors determining diabetes and impaired glucose tolerance: a 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diab Care 1995; 18: 1104–1112.
Hartweg J, Perera R, Montori V, Dinneen S, Neil A, Farmer A . Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus (Review). Cochrane Database Syst Rev 2008; 1: 1–60.
Neschen S, Morino K, Rossbacher J, Pongratz R, Cline G, Sono S et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes 2006; 55: 924–928.
Pérez-Matute P, Pérez-Echarri N, Martínez J, Marti A, Moreno-Aliaga M . Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-α. Br J Nutr 2007; 97: 389–398.
Rossi AS, Lombardo YB, Lacorte JM, Chicco AG, Rouault C, Slama G et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol Regul Integr Comp Physiol 2005; 289: R486–R494.
Burghardt P, Kemmerer E, Buck B, Osetek A, Yan C, Koch L et al. Dietary n-3:n-6 fatty acid ratios differentially influence hormonal signature in a rodent model of metabolic syndrome relative to healthy controls. Nutr Metab 2010; 7: 1–6.
Buettner R, Scholmerich J, Bollheimer L . High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity 2007; 15: 798–808.
Kondo K, Morino K, Nishio Y, Kondo M, Fuke T, Ugi S et al. Effects of a fish-based diet on the serum adiponectin concentration in young, non-obese, healthy Japanese subjects. J Atheroscler Thromb 2010; 17: 628–637.
Nomura S, Shouzu A, Omoto S, Inami N, Ueba T, Urase F et al. Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J Atheroscler Thromb 2009; 16: 83–90.
Gammelmark A, Madsen T, Varming K, Lundbye-Christensen S, Schmidt E . Low-dose fish oil supplementation increases serum adiponectin without affecting inflammatory markers in overweight subjects. Nutr Res 2012; 32: 15–23.
Patel JV, Lee KW, Tomson J, Dubb K, Hughes EA, Lip GY . Effects of omega-3 polyunsaturated fatty acids on metabolically active hormones in patients post-myocardial infarction. Int J Cardiol 2007; 115: 42–45.
Murata M, Kaji H, Takahashi Y, Iida K, Mizuno I, Okimura Y et al. Stimulation by eicosapentaenoic acids of leptin mRNA expression and its secretion in mouse 3T3-L1 adipocytes in vitro. Biochem Biophys Res Commun 2000; 270: 343–348.
Perez-Matute P, Marti A, Martinez JA, Fernandez-Otero MP, Stanhope KL, Havel PJ et al. Eicosapentaenoic fatty acid increases leptin secretion from primary cultured rat adipocytes: Role of glucose metabolism. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1682–R1688.
Mori TA, Burke V, Puddey IB, Shaw JE, Beilin LJ . Effect of fish diets and weight loss on serum leptin concentration in overweight, treated-hypertensive subjects. J Hypertens 2004; 22: 1983–1990.
Winnicki M, Somers VK, Accurso V, Phillips BG, Puato M, Palatini P et al. Fish-rich diet, leptin, and body mass. Circulation 2002; 106: 289–291.
Peyron-Caso E, Taverna M, Guerre-Millo Ml, se AVr, Pacher N, Slama Gr et al. Dietary (n-3) polyunsaturated fatty acids up-regulate plasma leptin in insulin-resistant rats. J Nutr 2002; 132: 2235–2240.
Rosicka M, Krsek M, Matoulek M, Jarkovska Z, Marek J, Justova V et al. Serum ghrelin levels in obese patients: the relationship to serum leptin levels and soluble leptin receptors levels. Physiol Res 2003; 52: 61–66.
Greenberg JA, Boozer CN . The leptin-fat ratio is constant, and leptin may be part of two feedback mechanisms for maintaining the body fat set point in non-obese male Fischer 344 rats. Horm Metab Res 1999; 31: 525–532.
Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005; 115: 3579–3586.
Ahima R, Kelly J, Elmquist J, Flier J . Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology 1999; 140: 4923–4931.
Itariu B, Zeyda M, Hochbrugger E, Neuhofer A, Prager G, Schindler K et al. Long-chain n−3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr 2012; 96: 1137–1149.
Koh K, Quon M, Shin K, Lim S, Lee Y, Sakuma I et al. Significant differential effects of omega-3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis 2012; 220: 537–544.
Neale E, Muhlhausler B, Probst Y, Batterham M, Fernandez F, Tapsell L . Short-term effects of fish and fish oil consumption on total and high molecular weight adiponectin levels in overweight and obese adults. Metabolism 2013; 62: 651–660.
Yannakoulia M, Yiannakouris N, Blüher S, Matalas A, Klimis-Zacas D, Mantzoros C . Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrino Metab 2003; 88: 1730–1736.
Yu Y, Zhu H . Chronological changes in metabolism and functions of cultured adipocytes: a hypothesis for cell aging in mature adipocytes. Am J Physiol Endocrinol Metab 2004; 286: E402–E410.
Koh E, Park J, Park H, Jeon M, Ryu J, Kim M et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 2007; 56: 2973–2981.
Choo H, Kim J, Kwon O, Lee C, Mun J, Han S et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 2006; 49: 784–791.
Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem 2001; 276: 41245–41254.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001; 50: 2094–2099.
Karbowska J, Kochan Z . Effect of DHEA on endocrine functions of adipose tissue, the involvement of PPARg. Biochem Pharmacol 2005; 70: 249–257.
van Vonderen M, van Agtmael M, Hassink E, Milinkovic A, Brinkman K, Geerlings S et al. Zidovudine/lamivudine for HIV-1 infection contributes to limb fat loss. PLoS One 2009; 4: e5647.
Addy C, Gavrila A, Tsiodras S, Brodovicz K, Karchmer A, Mantzoros C . Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrino Metab 2003; 88: 627–636.
Ferranti Sd, Mozaffarian D . The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 2008; 54: 945–955.
Pot G, Brouwer I, Enneman A, Rijkers G, Kampman E, Geelen A . No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: a randomized controlled trial in healthy, middle-aged individuals. Eu J Clin Nutr 2009; 63: 1353–1359.
Lo C-J, Chiu KC, Fu M, Lo R, Helton S . Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NFkB activity. J Surg Res 1999; 82: 216–221.
Ohata T, Fukuda K, Takahashi M, Sugimura T, Wakabayashi K . Suppression of nitric oxide production in lipopolysaccharide-stimulated macrophage cells by omega 3 polyunsaturated fatty acids. Jpn J Cancer Res 1997; 88: 234–237.
Khair-El-Din T, Sicher S, Vazquez M, Chung G, Stallworth K, Kitamura K et al. Transcription of the murine iNOS gene is inhibited by docosahexaenoic acid, a major constituent of fetal and neonatal sera as well as fish oils. J Exp Med 1996; 183: 1241–1246.
Endres S, Ghorbani R, Kelley V, Georgilis K, Lonnemann G, van der Meer J et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 1989; 320: 265–271.
Kremer J, Lawrence D, Jubiz W, Di Giacomo R, Rynes R, Bartholomew L et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. clinical and immunological effects. Arthritis Rheum 1990; 33: 810–820.
Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y . Docosahexaenoic and eisosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol 1997; 400B: 589–597.
Endres S . n-3 Polyunsaturated fatty acids and human cytokine synthesis. Lipids 1996; 31: S239–S242.
Meydani S, Endres S, Woods M, Goldin B, Soo C, Morrill L et al. Oral n-3 fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison in young and older women. J Nutr 1991; 121: 547–555.
Yudkin J, Stehouwer C, Emeis J, Coppack S . C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999; 19: 972–978.
Esposito K, Pontillo A, DiPalo C, Giugliano G, Masella M, Marfella R et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003; 289: 1799–1804.
Gnacińska M, Małgorzewicz S, Guzek M, Lysiak-Szydłowska W, Sworczak K . Adipose tissue activity in relation to overweight or obesity. Endokrynol Pol 2010; 61: 160–168.
Corica F, Allegra A, Corsonello A, Buemi M, Calapai G, Ruello A et al. Relationship between plasma leptin levels and the tumor necrosis factor-alpha system in obese subjects. Int J Obes (Lond) 1999; 23: 355–360.
Dedoussis G, Kapiri A, Samara A, Dimitriadis D, Lambert D, Pfister M et al. Expression of inflammatory molecules and associations with BMI in children. Eur J Clin Invest 2010; 40: 388–392.
van Dielen F, van’t Veer C, Schols A, Soeters P, Buurman W, Greve J . Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obesity 2001; 25: 1759–1766.
Zhang H, Kumar S, Barnett A, Eggo M . Tumor necrosis factor-a exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol 1999; 159: 79–88.
Kern P, Saghizadeh M, Ong J, Bosch RJ, Deem R, Simsolo RB . The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss and relationship to lipoprotein lipasa. J Clin Invest 1995; 95: 2111–2119.
Kirchgessen T, Uysal K, Wiesbrock S, Marino M, Hotamisligil G . Tumor necrosis factor-alpha contributes to obesityrelated hyperleptinemia by regulation leptin release from adipocytes. J Clin Invest 1997; 100: 2777–2782.
Schmidt E . Marine n-3 fatty acids and thrombosis. Thromb Res 2003; 111: 9–10.
Tomas E, Tsao T, Saha A, Murrey H, Zhang C, Itani S et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 2002; 99: 16309–16313.
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423: 762–769.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LV received funding from FIT Bioceuticals for the scholarship support of BG. These sponsors had no involvement in the collection, analysis or interpretation of the data; writing the report; or the decision to submit the paper for Publication. LV has received National Institute of Complementary Medicine and National Health and Medical Research Council of Australia competitive funding and Industry support for research into nutraceuticals.
Rights and permissions
About this article
Cite this article
Gray, B., Steyn, F., Davies, P. et al. Omega-3 fatty acids: a review of the effects on adiponectin and leptin and potential implications for obesity management. Eur J Clin Nutr 67, 1234–1242 (2013). https://doi.org/10.1038/ejcn.2013.197
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.197
Keywords
This article is cited by
-
Lipid profile after omega-3 supplementation in neonates with intrauterine growth retardation: a randomized controlled trial
Pediatric Research (2023)
-
Substitution of linoleic acid with α-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis
Scientific Reports (2018)
-
An orally-active adiponectin receptor agonist mitigates cutaneous fibrosis, inflammation and microvascular pathology in a murine model of systemic sclerosis
Scientific Reports (2018)
-
The effect of n-3 PUFAs on circulating adiponectin and leptin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials
Acta Diabetologica (2018)
-
Fish oil supplementation alleviates depressant-like behaviors and modulates lipid profiles in rats exposed to chronic unpredictable mild stress
BMC Complementary and Alternative Medicine (2015)