Abstract
BACKGROUND/OBJECTIVES:
Plasma 25-hydroxyvitamin D (P-25OHD) concentrations may affect pregnancy outcomes. To elucidate this further, we studied the effects of pre-conception P-25OHD concentrations on chances for pregnancy as well as the effects of P-25OHD during pregnancy on the risk of miscarriage, birth weight and length, Apgar score and head circumference. Moreover, we studied whether pregnancy and breastfeeding patterns affect maternal P-25OHD concentrations.
SUBJECTS/METHODS:
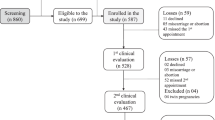
A total of 153 healthy Caucasian women with pregnancy plans were followed with measurements performed before pregnancy, at pregnancy weeks 11±2, 22±1 and 35±2 as well as 15±7, 129±12 and 280±15 days postpartum. Furthermore, 75 non-pregnant, age-matched women were followed in parallel as controls.
RESULTS:
The 203 women were aged 29 (25–35) years. At baseline, median P-25OHD was 59 nmol/l. Of these women, 31% had P-25OHD <50 nmol/l, whereas 12% had levels above 80 nmol/l. Within ∼6 months after inclusion, 63% conceived. P-25OHD was not associated with chances of conceiving or overall risk of miscarriage. However, women with a miscarriage in their second trimester (n=3) had lower P-25OHD concentrations at measurements performed in the first trimester compared with women without a miscarriage (P=0.03). P-25OHD before or during pregnancy was not associated with gestational length or infant parameters. Adjustments for possible confounders did not change the result. During pregnancy, P-25OHD changed significant over time, but similar changes occurred within the control group, indicating no effect of pregnancy per se (P=0.59). Overall, P-25OHD did not differ according to length of breastfeeding at 2 weeks, and 4 and 9 months postpartum, although women breastfeeding for >9 months had lower P-25OHD levels at the last visit compared with the controls.
CONCLUSION:
P-25OHD concentrations did not affect fertility or pregnancy outcomes, although low P-25OHD may be associated with an increased risk of late miscarriage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Johnson LE, DeLuca HF . Reproductive defects are corrected in vitamin d-deficient female rats fed a high calcium, phosphorus and lactose diet. J Nutr 2002; 132, 2270–2273.
Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA 2001; 98, 7498–7503.
Blomberg JM, Nielsen JE, Jorgensen A, Rajpert-De ME, Kristensen DM, Jorgensen N et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod 2010; 25, 1303–1311.
Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M et al. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25 (OH) 2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol 2009; 7, 140.
Lips P . Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001; 22, 477–501.
Holick MF . Vitamin D deficiency. N Engl J Med 2007; 357, 266–281.
Leffelaar ER, Vrijkotte TG, van EM . Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr 2010; 104, 108–117.
Moller UK, Ramlau-Hansen CH, Rejnmark L, Heickendorff L, Henriksen TB, Mosekilde L . Postpartum vitamin D insufficiency and secondary hyperparathyroidism in healthy Danish women. Eur J Clin Nutr 2006; 60, 1214–1221.
Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N et al. Low maternal Vitamin D status and fetal bone development: cohort study. J Bone Miner Res 2010; 25, 14–19.
Stene LC, Ulriksen J, Magnus P, Joner G . Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia 2000; 43, 1093–1098.
Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr 2007; 85, 853–859.
Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy 2009; 39, 875–882.
McGrath J, Barnett A, Eyles D, Burne T, Pedersen CB, Mortensen PB . The impact of nonlinear exposure-risk relationships on seasonal time-series data: modelling Danish neonatal birth anthropometric data. BMC Med Res Methodol 2007; 7, 45.
Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F . Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab 2000; 85, 1828–1833.
Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B . Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev 2008; 24, 27–32.
Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr 2010; 140, 999–1006.
Morley R, Carlin JB, Pasco JA, Wark JD, Ponsonby AL . Maternal 25-hydroxyvitamin D concentration and offspring birth size: effect modification by infant VDR genotype. Eur J Clin Nutr 2009; 63, 802–804.
Watson PE, McDonald BW . The association of maternal diet and dietary supplement intake in pregnant New Zealand women with infant birthweight. Eur J Clin Nutr 2010; 64, 184–193.
Scholl TO, Chen X . Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev 2009; 85, 231–234.
Sayers A, Tobias JH . Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab 2009; 94, 765–771.
Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006; 367, 36–43.
Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 2008; 62, 68–77.
Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I . Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr 2009; 98, 1360–1362.
Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J 1980; 280, 751–754.
Sabour H, Hossein-Nezhad A, Maghbooli Z, Madani F, Mir E, Larijani B . Relationship between pregnancy outcomes and maternal vitamin D and calcium intake: a cross-sectional study. Gynecol Endocrinol 2006; 22, 585–589.
Holmes VA, Barnes MS, Alexander HD, McFaul P, Wallace JM . Vitamin D deficiency and insufficiency in pregnant women: a longitudinal study. Br J Nutr 2009; 102, 876–881.
Hillman LS, Slatopolsky E, Haddad JG . Perinatal vitamin D metabolism. IV. Maternal and cord serum 24, 25-dihydroxyvitamin D concentrations. J Clin Endocrinol Metab 1978; 47, 1073–1077.
Kent GN, Price RI, Gutteridge DH, Smith M, Allen JR, Bhagat CI et al. Human lactation: forearm trabecular bone loss, increased bone turnover, and renal conservation of calcium and inorganic phosphate with recovery of bone mass following weaning. J Bone Miner Res 1990; 5, 361–369.
Moller UK, Vieth SS, Mosekilde L, Rejnmark L . Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int 2011; e-pub ahead of print 25 May 2011.
Maunsell Z, Wright DJ, Rainbow SJ . Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem 2005; 51, 1683–1690.
Hojskov CS, Heickendorff L, Moller HJ . High-throughput liquid-liquid extraction and LCMSMS assay for determination of circulating 25 (OH) vitamin D3 and D2 in the routine clinical laboratory. Clin Chim Acta 2010; 411, 114–116.
Rowe T . Fertility and a woman's age. J Reprod Med 2006; 51, 157–163.
Axmon A, Rylander L, Albin M, Hagmar L . Factors affecting time to pregnancy. Hum Reprod 2006; 21, 1279–1284.
Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM . Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 2007; 92, 3517–3522.
Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 2009; 20, 720–726.
Morley R, Carlin JB, Pasco JA, Wark JD . Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 2006; 91, 906–912.
Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A et al. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol 2008; 6, 3.
Evans KN, Bulmer JN, Kilby MD, Hewison M . Vitamin D and placental-decidual function. J Soc Gynecol Investig 2004; 11, 263–271.
Mannion CA, Gray-Donald K, Koski KG . Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ 2006; 174, 1273–1277.
Marya RK, Rathee S, Lata V, Mudgil S . Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest 1981; 12, 155–161.
Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H . Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol 1986; 68, 300–304.
Brunvand L, Quigstad E, Urdal P, Haug E . Vitamin D deficiency and fetal growth. Early Hum Dev 1996; 45, 27–33.
Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL . Vitamin D supplementation during pregnancy: double blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011; 26, 2341–2357.
Mortensen LH, Diderichsen F, Smith GD, Andersen AM . The social gradient in birthweight at term: quantification of the mediating role of maternal smoking and body mass index. Hum Reprod 2009; 24, 2629–2635.
Oken E, Osterdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strom M et al. Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish national birth cohort. Am J Clin Nutr 2008; 88, 789–796.
Mosekilde L . Vitamin D and the elderly. Clin Endocrinol 2005; 62, 265–281.
Acknowledgements
We are grateful for the financial support provided to the project from The Danish Agency for Science, Technology and Innovation, The Aarhus University Research Foundation, AP Moeller Foundation for the Advancement of Medical Science, Svend Fældings Humanitære Fond, The Lundbeck Foundation, Aarhus University Fellowship and Helga and Peter Kornings Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Møller, U., Streym, S., Heickendorff, L. et al. Effects of 25OHD concentrations on chances of pregnancy and pregnancy outcomes: a cohort study in healthy Danish women. Eur J Clin Nutr 66, 862–868 (2012). https://doi.org/10.1038/ejcn.2012.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2012.18
Keywords
This article is cited by
-
Vitamin D in pregnancy (GRAVITD) – a randomised controlled trial identifying associations and mechanisms linking maternal Vitamin D deficiency to placental dysfunction and adverse pregnancy outcomes – study protocol
BMC Pregnancy and Childbirth (2023)
-
Perikonzeptioneller Einfluss von Ernährung und Mikronährstoffen auf die Reproduktionsfunktion
Gynäkologische Endokrinologie (2022)
-
Vitamin D during pregnancy and its association with birth outcomes: a Brazilian cohort study
European Journal of Clinical Nutrition (2021)
-
New aspects of vitamin D metabolism and action — addressing the skin as source and target
Nature Reviews Endocrinology (2020)
-
Serum vitamin D and vitamin D-binding protein levels in mother-neonate pairs during the lactation period
Italian Journal of Pediatrics (2018)