Abstract
Background/Objectives:
Vitamin D mediates immunomodulatory functions, and its deficiency has been associated with an increased prevalence of immunological diseases. The supplementation of vitamin D might be therapeutically beneficial, for example, in lupus erythematosus patients. However, its affect on established recall immune responses is undefined.
Subjects/Methods:
In all, 32 individuals were randomized in a placebo controlled, double-blind setting, and received vitamin D (daily 2000 IU) for 10 weeks followed by tetanus toxoid (TT) booster immunization.
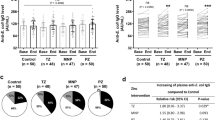
Results:
During vitamin D supplementation the median 25-hydroxyvitamin D serum concentration increased to 80.3 nM, which as expected decreased in the placebo group to 29.1 nM during the ultraviolet-deprived winter months. The TT-specific immunoglobulin G (IgG) boost efficiency was marginal higher in the vitamin D group (P=0.04). The increase of the 25-hydroxyvitamin D levels correlated with the increase of TT–IgG serum concentrations. The induction of specific serum IgA and specific antibody secreting cells was comparable between both groups. Accordingly, the TT-specific and polyclonally triggered T-cell cytokine profiles were stable as well.
Conclusions:
Vitamin D supplementation was successful and booster immunization induced efficiently specific antibodies titers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Adams JS, Hewison M (2008). Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4, 80–90.
Adorini L, Penna G (2008). Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 4, 404–412.
Daniel C, Schlauch T, Zugel U, Steinmeyer A, Radeke HH, Steinhilber D et al. (2005). 22-ene-25-oxa-vitamin D: a new vitamin D analogue with profound immunosuppressive capacities. Eur J Clin Invest 35, 343–349.
De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S et al. (2004). Vaccination in humans generates broad T cell cytokine responses. J Immunol 173, 5372–5380.
Enioutina EY, Bareyan D, Daynes RA (2008). TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine 26, 601–613.
Enioutina EY, Visic D, McGee ZA, Daynes RA (1999). The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine 17, 3050–3064.
Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M et al. (2005). Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 11, 1118–1124.
Frolich D, Giesecke C, Mei HE, Reiter K, Daridon C, Lipsky PE et al. (2010). Secondary immunization generates clonally related antigen-specific plasma cells and memory B cells. J Immunol 185, 3103–3110.
Hayes CE, Nashold FE, Spach KM, Pedersen LB (2003). The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 49, 277–300.
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ (2003). Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77, 204–210.
Heine G, Anton K, Henz BM, Worm M (2002). 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol 32, 3395–3404.
Heine G, Niesner U, Chang HD, Steinmeyer A, Zugel U, Zuberbier T et al. (2008). 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol 38, 2210–2218.
Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C (2008). Vitamin D status and health correlates among German adults. Eur J Clin Nutr 62, 1079–1089.
Holick MF (2007). Vitamin D deficiency. N Engl J Med 357, 266–281.
Ivanov AP, Dragunsky EM, Chumakov KM (2006). 1,25-dihydroxyvitamin D3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J Infect Dis 193, 598–600.
Mei HE, Yoshida T, Sime W, Hiepe F, Thiele K, Manz RA et al. (2008). Blood-borne human plasma cells in steady-state are derived from mucosal immune responses. Blood 113, 2461–2469.
Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G et al. (2005). Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105, 1614–1621.
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R (2006). Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83, 754–759.
Van der Stede Y, Cox E, Van den broeck W, Goddeeris BM (2001). Enhanced induction of the IgA response in pigs by calcitriol after intramuscular immunization. Vaccine 19, 1870–1878.
Vieth R (1999). Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 69, 842–856.
Vieth R (2007). Vitamin D toxicity, policy, and science. J Bone Miner Res 22 (Suppl 2), V64–V68.
Zittermann A, Dembinski J, Stehle P (2004). Low vitamin D status is associated with low cord blood levels of the immunosuppressive cytokine interleukin-10. Pediatr Allergy Immunol 15, 242–246.
Acknowledgements
We thank Dr Helmut Orawa and Ramona Scheuffele (both Institut für Biometrie und klinische Epidemiologie, Charité Campus Mitte) for statistical assistance and Dennis Ernst for outstanding technical assistance. This work was supported by the Deutsche Forschungsgesellschaft (DFG–SFB650/TP5 and TP16) and by the Charité–Universitätsmedizin Berlin. GH and GD were supported by the DFG–SFB650 TP5 and HM by the DFG–SFB650/TP16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Heine, G., Drozdenko, G., Lahl, A. et al. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur J Clin Nutr 65, 329–334 (2011). https://doi.org/10.1038/ejcn.2010.276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2010.276
Keywords
This article is cited by
-
Vitamin D and the hepatitis B vaccine response: a prospective cohort study and a randomized, placebo-controlled oral vitamin D3 and simulated sunlight supplementation trial in healthy adults
European Journal of Nutrition (2021)
-
Vitamin D status predicts reproductive fitness in a wild sheep population
Scientific Reports (2016)
-
Serum vitamin D levels are positively associated with varicella zoster immunity in chronic dialysis patients
Scientific Reports (2014)
-
Relatively high serum vitamin D levels do not impair the antibody response to encapsulated bacteria
European Journal of Clinical Microbiology & Infectious Diseases (2013)