Abstract
We have proposed a new method which can be applied in assessing the intracellular production of hydrogen peroxide. Using this assay we have examined the hydrogen peroxide generation during the L-glutamate induced oxidative stress in the HT22 hippocampal cells. The detection of hydrogen peroxide is based on two crucial reagents cis-[Cr(C2O4)(pm)(OH2)2]+ (pm denotes pyridoxamine) and 2-ketobutyrate. The results obtained indicate that the presented method can be a promising tool to detect hydrogen peroxide in biological samples, particularly in cellular experimental models.
Similar content being viewed by others
Introduction
Reactive oxygen species (ROS) are generated in cells under normal conditions e.g. during aerobic respiration1. Electrons transferred in the mitochondrial electron transport chain may escape and interact with molecular oxygen to form superoxide anion ( )1. Superoxide dysmutase (SOD) catalyzes dismutation of
)1. Superoxide dysmutase (SOD) catalyzes dismutation of  resulting in the generation of hydrogen peroxide (H2O2), which in turn can react with Fe2+ in the Fenton reaction to form hydroxyl radicals (HO·)2,3,4. When the cellular antioxidant defense mechanisms fail to effectively eliminate ROS the oxidative stress appears. Superoxide anion, hydroxyl radical or hydrogen peroxide lead to irreversible damage of lipids, proteins and DNA, resulting in dysfunction of cell organelles5,6. The extraordinary high rate of respiration in some organs such as the brain makes them particularly susceptible to ROS mediated injury. ROS and induced by them oxidative damage are believed to contribute to the development of many diseases such as cancer, arteriosclerosis, diabetes and neurodegenerative diseases7,8,9,10. Establishing the role of oxidative stress requires the ability to measure its mediators accurately11. Therefore, there is a need to develop and improve sensitive and specific methods to detect and evaluate the level of reactive oxygen species in biological samples of different origin. A method of protecting the neuronal cells HT22 against the oxidative stress generated by hydrogen peroxide has been investigated and described by Jia and co-workers12. This method is based on cytoprotection properties of endogenous cannabinoid anandamide. Moreover, recently it has been found that the Homer1a - protein regulating calcium pathways are involved in the glutamate-induced HT22 cell death13. Homer1a plays the cytoprotective role in the prevention of the glutamate-induced oxidative injury. In most cases existing methods to detect hydrogen peroxide in biological samples are based on the horseradish peroxidase (HRP) mediated reaction14. These methods are sensitive but have also disadvantages because HRP can react with many cellular substrates. Fluorescent probes used for the detection of H2O2 under biological conditions in addition to the advantages have some limitations. For example some probes require long response times (30–60 min) or an increase in the luminescence is also observed in the presence of the citrate and phosphate buffer15. Moreover, the autofluorescence of cells is a problem in the flow cytometry technique or fluorescence microscopy for the detection of H2O2. The purpose of this study was to design a method for the hydrogen peroxide detection which is characterized by a high sensitivity, moreover the autofluorescence of samples is eliminated and the results obtained by this method can be presented in concentration units.
resulting in the generation of hydrogen peroxide (H2O2), which in turn can react with Fe2+ in the Fenton reaction to form hydroxyl radicals (HO·)2,3,4. When the cellular antioxidant defense mechanisms fail to effectively eliminate ROS the oxidative stress appears. Superoxide anion, hydroxyl radical or hydrogen peroxide lead to irreversible damage of lipids, proteins and DNA, resulting in dysfunction of cell organelles5,6. The extraordinary high rate of respiration in some organs such as the brain makes them particularly susceptible to ROS mediated injury. ROS and induced by them oxidative damage are believed to contribute to the development of many diseases such as cancer, arteriosclerosis, diabetes and neurodegenerative diseases7,8,9,10. Establishing the role of oxidative stress requires the ability to measure its mediators accurately11. Therefore, there is a need to develop and improve sensitive and specific methods to detect and evaluate the level of reactive oxygen species in biological samples of different origin. A method of protecting the neuronal cells HT22 against the oxidative stress generated by hydrogen peroxide has been investigated and described by Jia and co-workers12. This method is based on cytoprotection properties of endogenous cannabinoid anandamide. Moreover, recently it has been found that the Homer1a - protein regulating calcium pathways are involved in the glutamate-induced HT22 cell death13. Homer1a plays the cytoprotective role in the prevention of the glutamate-induced oxidative injury. In most cases existing methods to detect hydrogen peroxide in biological samples are based on the horseradish peroxidase (HRP) mediated reaction14. These methods are sensitive but have also disadvantages because HRP can react with many cellular substrates. Fluorescent probes used for the detection of H2O2 under biological conditions in addition to the advantages have some limitations. For example some probes require long response times (30–60 min) or an increase in the luminescence is also observed in the presence of the citrate and phosphate buffer15. Moreover, the autofluorescence of cells is a problem in the flow cytometry technique or fluorescence microscopy for the detection of H2O2. The purpose of this study was to design a method for the hydrogen peroxide detection which is characterized by a high sensitivity, moreover the autofluorescence of samples is eliminated and the results obtained by this method can be presented in concentration units.
The new method we propose is based on the following steps: (a) the nonenzymatic reaction of 2-ketobutyrate with H2O2 resulting in the CO2 release and (b) the interaction of the coordination compound cis-[Cr(C2O4)(pm)(OH2)2]+ with CO2. 2-ketobutyrate reacts with H2O2 with 1:1 stoichiometry (1). The amount of H2O2 in this reaction is equivalent with the CO2 released. 2-ketoacids such as 2-ketobutyrate or pyruvate can react nonenzymatically with H2O2, yielding CO2, H2O and the corresponding one carbon atom shorter carboxylic acid. This reaction was first reported by Holleman16.

Thus, based on the CO2 measurement it is possible to assess the level of H2O2. The coordination complex of chromium [Cr(C2O4)(pm)(OH2)2]+, where pm denotes pyridoxamine, was shown to effectively catch CO217. Previously we successfully used cis-[Cr(C2O4)(pm)(OH2)2]+ as a molecular biosensor to detect CO2 in osteosarcoma 143B cells treated with hydrogen peroxide18. In this study we demonstrate an improved version of this method based on both cis-[Cr(C2O4)(pm)(OH2)2]+ and 2-ketobutyrate.
Results
To study the generation of hydrogen peroxide in a cellular model we used hippocampal HT22 cells treated with 5 mM L-glutamate for 24 h. Glutamate belongs to neurotransmitters of the central nervous system. However, at high (mM) concentration it was found to induce death of neurons19. 5 mM glutamate was reported to induce oxidative stress in HT22 cells resulting in cell death20. Our results also showed that the viability of HT22 cells treated with 5 mM L-glutamate for 24 h dramatically decreases (publication in press). On the basis of our proposed hydrogen peroxide detection method, we measured CO2 concentration after adding of 2-ketobutyrate. 2-ketobutyrate reacts with H2O2. The amount of CO2 released in this reaction reflects the amount of H2O2. In addition to cell lysates, the CO2 level was assessed in supernatants as well as in cell free culture medium – all of them potentially contained H2O2. It is important to note that they differed in chemical composition. In order to determine the most effective concentration of 2-ketobutyrate required to scavenge H2O2 we tested its following concentrations: 0.5 mM, 1 mM, 2 mM, 3 mM, 4 mM, 5 mM, 6 mM, 7 mM, 8 mM, 9 mM, 10 mM, 11 mM, 12 mM, 13 mM, 14 mM and 15 mM.
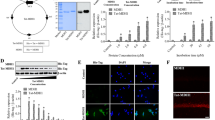
In order to assess the CO2 concentration in samples we used a specific molecular biosensor cis-[Cr(C2O4)(pm)(OH2)2]+ and the spectrophotometric stopped-flow technique. The structure of the synthesized complex of chromium(III), cis-[Cr(C2O4)(pm)(OH2)2]+ ion, is shown in Fig. 121.
It turned out that the coordination ion, cis-[Cr(C2O4)(pm)(OH2)2]+, could be successfully applied in the case of the detection of carbon dioxide generated in the reaction of decarboxylation of 2-ketobutyrate caused by H2O2. This reaction between the Cr(III) complex ion with pyridoxamine and the carbon dioxide was observed between 340–700 nm by using spectrophotometric stopped-flow method. The concentration of carbon dioxide has been identified based on the method which was used and described in the paper22.
As shown in Fig. 2 the CO2 levels in lysates of cells incubated in the presence or absence of 5 mM L-glutamate, both measured without the addition of 2-ketobutyrate, were 0.025 μmol mg−1 protein (±0.006) and 0.006 μmol mg−1 protein (±0.002), respectively. We found that after addition of 0.5–10 mM 2-ketobutyrate to the lysates of L-glutamate-treated HT22 cells, the CO2 level gradually increased (Fig. 2). Noteworthy, at 2-ketobutyrate concentrations ranging from 10 mM to 15 mM the CO2 level was very similar (ranging from 0.14 μmol mg−1 protein to 0.15 μmol mg−1 protein). Moreover, the highest CO2 level was then observed. CO2 was also detected in lysates of cells incubated in the absence of 5 mM L-glutamate. However, its level was much lower than that in the presence of L-glutamate.
HT22 cells were incubated for 24 h in the presence or absence of 5 mM sodium L-glutamate. After treatment, cells were lysed. Before the CO2 measurement sodium 2-ketobutyrate was added (final concentations: 0.5–15 mM, respectively). The CO2 level was determined using cis-[Cr(C2O4)(pm)(OH2)2]+ and a stopped-flow technique. Data are presented as mean ± SD of three independent experiments. *p < 0.05, ***p < 0.001, statistically significant differences between samples incubated in the presence and samples incubated in the absence of 5 mM L-glutamate.
Intracellularly produced H2O2 has the ability to penetrate biological membranes and affect neighbouring cells23. In order to better evaluate and understand the chemical environment of cells in our experimental model, we assessed the CO2 level in supernatants. The results revealed that the CO2 levels in supernatants separated from cells incubated in the presence or absence of 5 mM L-glutamate, both measured without the addition of 2-ketobutyrate, were 1.315 μM (±0.220) and 0.949 μM (±0.114), respectively (Fig. 3). We found that the CO2 level gradually increased depending on the concentration of 2-ketobutyrate added (Fig. 3). In the case of supernatants separated from cells incubated in the presence of L-glutamate, at 2-ketobutyrate concentrations ranging from 10 mM to 15 mM the CO2 level was very similar and the highest (ranging from 7.15 μM to 7.6 μM). Moreover, the concentration of CO2 detected in supernatants separated from cells incubated in the absence of L-glutamate was then much lower than that in the presence of L-glutamate.
HT22 cells were incubated for 24 h in the presence or absence of 5 mM sodium L-glutamate. After treatment, supernatants were separated from cells. Before the CO2 measurement sodium 2-ketobutyrate was added (final concentations: 0,5–15 mM, respectively). The CO2 level was determined using cis-[Cr(C2O4)(pm)(OH2)2]+ and a stopped-flow technique. Data are presented as mean ± SD of three independent experiments. *p < 0.05, statistically significant differences between samples incubated in the presence and samples incubated in the absence of 5 mM L-glutamate.
The oxidation of chemical components of the culture medium may be a source of hydrogen peroxide generation24,25. The source of hydrogen peroxide in the cell-free DMEM medium, used in this study, in the absence of L-glutamate could be the oxidation of some chemical components of the culture medium. It has been reported that thiol compounds such as cysteine are unstable in culture media and can oxidize to generate hydrogen peroxide24. Noteworthy, cysteine is a constituent of the DMEM medium. There is also evidence that riboflavin may be a source of the photogeneration of ROS in cell culture media25. It has been shown that it is possible to generate superoxide anion and hydrogen peroxide as a product of its dismutation. Taking it into consideration, we have examined the CO2 level in the cell-free complete culture medium. The CO2 concentration in medium samples incubated in the presence or absence of 5 mM L-glutamate, both measured without the addition of 2-ketobutyrate, were 1.153 μM (±0.206) and 0.998 μM (±0.068), respectively (Fig. 4). We found that upon addition of 2-ketobutyrate at increasing concentrations, the CO2 level gradually increased (Fig. 4). The increase in CO2 concentration was observed in medium samples incubated in the presence or absence of 5 mM L-glutamate. Noteworthy, in the presence of L-glutamate after adding of 2-ketobutyrate at concentrations ranging from 12 mM to 15 mM the CO2 level was the highest and nearly constant (about 4.5 μM). In the absence of L-glutamate, at 2-ketobutyrate concentrations ranging from 6 mM to 15 mM only slight changes in the CO2 concentration appeared (ranging from 3.6 μM to 3.8 μM).
The cell-free culture medium was incubated for 24 h in the presence or absence of 5 mM sodium L-glutamate. After treatment, sodium 2-ketobutyrate was added (final concentations: 0.5–15 mM, respectively). The CO2 level was determined using cis-[Cr(C2O4)(pm)(OH2)2]+ and a stopped-flow technique. Data are presented as mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 statistically significant differences between samples incubated in the presence and samples incubated in the absence of 5 mM L-glutamate.
Discussion
In this study, we demonstrate a new method of hydrogen peroxide detection. It can be applied to assess hydrogen peroxide level in different biological samples, e.g. cell lysates, supernatants or cell-free culture medium.
Our results suggest that the 10 mM–15 mM concentration range of 2-ketobutyrate can be used in the proposed method to effectively detect and assess the CO2 concentration in lysates of 5 mM L-glutamate-treated HT22 cells. At this concentration range the CO2 level was nearly the same. The main source of CO2 in cell lysates was then the reaction of 2-ketobutyrate with intracellularly produced H2O2. In order to find out whether the cell metabolism may affect the CO2 level, we incubated HT22 cells in the absence of 5 mM L-glutamate. We assume that in our experimental model CO2, whose main source are metabolic pathways, can be detected in the absence of L-glutamate (in the absence of H2O2 generating agents) and simultaneously in the absence of 2-ketobutyrate. Under these conditions CO2 does not originate from the reaction of 2-ketobytyrate with H2O2 as well as is not affected by L-glutamate, hence its main source can probably be metabolic pathways. Our results have shown that the CO2 level in lysates of cells incubated in the absence of 5 mM L-glutamate, measured without the addition of 2-ketobutyrate, is 0.006 μmol mg−1 protein (±0.002). It reflects the background CO2 level for lysates of cells nontreated with L-glutamate. The CO2 level measured in the absence of L-glutamate, but after the addition of 2-ketobutyrate includes CO2 originating from metabolic pathways. Another source can be endogenous H2O2 related to the function of some cell organelles, e.g. peroxisomes, mitochondria. Noteworthy, the CO2 level in lysates of cells incubated in the presence of 5 mM L-glutamate, measured without the addition of 2-ketobutyrate, is 0.025 μmol mg−1 protein (±0.006). It may be considered as the background CO2 level for lysates of cells treated with L-glutamate. It appears slightly higher than in the absence of 5 mM L-glutamate suggesting that 5 mM L-glutamate may affect the CO2 level. The background signal may be a disadvantage of the proposed method. However, a similar problem or disadvantage related to the background signal exists in many other experimental methods. Cellular autofluorescence, for example, resulting from natural fluorescence properties of some structural components of cells, affects the sensitivity of flow cytometry assays26,27,28. Autofluorescence of cells or tissues is also a problem in another widely used technique such as fluorescence microscopy29,30. The background fluorescence interferes with detection of specific fluorescent signals. In our proposed method the problem related to the background CO2 level in cell lysates can be solved. The possible solution could be to subtract the background signal from the analysis.
The results indicated that, the 10 mM–15 mM concentration range of 2-ketobutyrate is sufficient to assess the CO2 level in supernatants separated from 5 mM L-glutamate-treated HT22 cells. The CO2 level in the samples measured was then similar and stopped increasing. As mentioned previously, the main source of CO2 in supernatants was H2O2 released from the cells where it was produced23. However, the additional source of CO2 can also be H2O2 generated during oxidation of chemical components of the culture medium24,25. Therefore, we also determined the CO2 level in supernatants separated from cells incubated in the absence of 5 mM L-glutamate. We noticed that it was much lower than in the presence of 5 mM L-glutamate. CO2 detected in supernatants separated from cells nontreated with 5 mM L-glutamate, measured without the addition of 2-ketobutyrate, is 0.949 μM (±0.114) and corresponds to the background CO2 level in supernatants separated from cells nontreated with 5 mM L-glutamate. It results mostly from the oxidation of chemical components of the culture medium. CO2 detected in the supernatant separated from cells treated with 5 mM L-glutamate, measured without the addition of 2-ketobutyrate, is 1.315 μM (±0.220) and reflects the background CO2 level in supernatants separated from cells treated with 5 mM L-glutamate. Its source is related not only to the oxidation of chemical components of the culture medium, but also to the effects of L-glutamate on culture medium components.
We found that 2-ketobutyrate at a concentration from 12 mM to 15 mM can be used to assess the CO2 level in the cell-free culture medium incubated with 5 mM L-glutamate. The CO2 concentration was then about 0.8 μM higher in the presence of L-glutamate than in its absence, suggesting that 5 mM L-glutamate may generate a small amount of H2O2 and/or CO2 in the culture medium. The CO2 level (corresponding to H2O2) in the presence of L-glutamate reflects the oxidation of components of the culture medium as well as the influence of L-glutamate on culture medium components. The CO2 level measured without the addition of 2-ketobutyrate can be considered as the background CO2 level in the cell-free culture medium. In the presence of 5 mM L-glutamate it is 1.153 μM (±0.206), whereas in the absence of 5 mM L-glutamate 0.998 μM (±0.068). The background CO2 level may result mainly from the generation of CO2 during the oxidation/decomposition of some its components. The CO2 level in the presence of L-glutamate as well as the CO2 level in the absence of L-glutamate, both measured after addition of 2-ketobutyrate, include the background CO2 level. However, it is important to note that the CO2 level measured in cell lysates should not be affected by the background CO2 in culture medium/supernatants. Before analysis, cells are separated from the culture medium, washed and then lysed to obtain cell lysates.
It is important to note that cell lysates, supernatants and cell-free culture medium differ in chemical composition. Thus, they should be considered examples of different experimental models. The chemical composition of culture media is considered to resemble (at least in part) the natural cellular environment. It should imitate physiological conditions. However, further studies are needed to find out whether the method presented here can be applied to detect hydrogen peroxide in biological fluids, e.g. in the blood serum. Our results revealed that the concentration range of 2-ketobutyrate needed to assess the CO2 level in different models may not be the same. This highlights the importance of preliminary studies required to establish experimental conditions in every case. However, it is not a big disadvantage, because in many experiments it is a common procedure, e.g. a standard curve in protein measurements.
Conclusions
The new method we propose can be useful in comparative studies, e.g. unknown samples can be compared to control samples. An advantage of this method is its high sensitivity. The lower limit of detection is equal to 10−7 M22. Another advantage of the method is that the results can be presented in concentration units. Majority of methods allow to use arbitrary instead of more precise concentration units, e.g. flow cytometry assays using 2′,7′- dichlorofluorescin diacetate or hydroethidine to measure intracellular production of reactive oxygen species31,32. In conclusion, the presented method based on both cis-[Cr(C2O4)(pm)(OH2)2]+ and 2-ketobutyrate seems to be a very promising tool to study the hydrogen peroxide generation.
Methods
Chemicals
Reagents: sodium 2-ketobutyrate and L-glutamic acid monosodium salt monohydrate were purchased from Sigma (USA). L-glutamic acid monosodium salt solutions were prepared in the sterile physiological saline solution before use. Each time sodium 2-ketobutyrate solutions before use were prepared in sterile water. Cis-[Cr(C2O4)(pm)(OH2)2]+ ion was synthesized according to standard literature procedures. The final product - cis-[Cr(C2O4)(L-L)(O2CO)]− (L-L denotes bidentate ligand – pyridoxamine (pm)) was prepared by the known method described in the literature17.
Cell culture
The mouse hippocampal neuronal HT22 cell line was kindly provided by Professor T. Grune (Institute of Biological Chemistry and Nutrition, University Hohenheim, Stuttgart). HT22 cells were maintained in CO2 incubator, at 37 °C in a humidified atmosphere containing 5% CO2. Cells were cultured in Dulbecco’s Modified Eagle’s Medium without sodium pyruvate (DMEM, Sigma-Aldrich, USA), supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, USA), 100 IU mL-1 penicillin (Sigma-Aldrich, USA) and 100 μg/mL streptomycin (Sigma-Aldrich, USA).
Cell treatment and CO2 measurement
HT22 cells were seeded onto 6-well plates (2 × 105 cells per well) and allowed to attach for 24 h. Cells were then incubated for 24 h in the presence or absence of 5 mM sodium L-glutamate. Additionally, the cell-free culture medium (DMEM) was incubated for 24 h with or without 5 mM sodium L-glutamate. After incubation, cells as well as cell culture medium in which the cells were incubated were collected and separated by centrifugation (300 × g, 5 min, room temperature). Next, HT22 cells were washed with PBS and suspended in a lysis buffer (0.15 M NaCl, 0.005 M EDTA, 1% Triton X-100, 0.01 M Tris-HCl). The cell-free complete culture medium was also collected. Sodium 2-ketobutyrate solution was then added (final concentration: 0.5 mM, 1 mM, 2 mM, 3 mM, 4 mM, 5 mM, 6 mM, 7 mM, 8 mM, 9 mM, 10 mM, 11 mM, 12 mM, 13 mM, 14 mM, 15 mM) to the samples (cell lysates, supernatants, cell-free culture medium), prior to CO2 measurement. The protein level in cell lysates was measured using Pierce BCATM Protein Assay Kit (Thermo Scientific, USA) according to the manufacturer’s instruction. The CO2 concentration was assessed using a spectrophotometric stopped-flow technique and a coordinate cis-[Cr(C2O4)(pm)(OH2)2]+ ion as a molecular biosensor of CO2.
Statistical analysis
Statistical analysis was performed using the Statistica 9 software (StatSoft, Poland). Data are expressed as mean ± SD. Statistical differences were evaluated using the Mann-Whitney U test. Differences were considered significant at p < 0.05, p < 0.01, p < 0.001, respectively.
Additional Information
How to cite this article: Jacewicz, D. et al. Method for detection of hydrogen peroxide in HT22 cells. Sci. Rep. 7, 45673; doi: 10.1038/srep45673 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Grivennikova, V. G. & Vinogradov, A. D. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 1757, 553–561 (2006).
McCord, J. M. & Fridovich, I. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 244, 6049–6055 (1969).
Boveris, A. & Chance, B. The mitochondrial generation of hydrogen peroxide General properties and effect of hyperbaric oxygen. Biochem J. 134, 707–716 (1973).
Fenton, H. J. H. Oxidation of tartaric acid in presence of iron. J Chem Soc. 65, 899–910 (1894).
Pranczk, J., Jacewicz, D., Wyrzykowski, D. & Chmurzynski, L. Analytical methods for determination of reactive oxygen species. Curr. Pharm. Anal. 10, 293–304 (2014).
Wyrzykowski, D., Inkielewicz-Stępniak, I., Pranczk, J., Żamojć, K., Zięba, P., Tesmar, A., Jacewicz D., Ossowski, T. & Chmurzyński, L. Physicochemical properties of ternary oxovanadium(IV) complexes with oxydiacetate and 1, 10-phenanthroline or 2, 2′-bipyridine. Cytoprotective activity in hippocampal neuronal HT22 cells. BioMetals 28, 307–320 (2015).
Friedman, J., Peleg, E., Kagan, T., Shnizer, S. & Rosenthal, T. Oxidative stress in hypertensive, diabetic, and diabetic hypertensive rats. Am. J. Hypertens. 16, 1049–1052 (2003).
Reynolds, A., Laurie, C., Mosley, R. L. & Gendelman, H. E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 82, 297–325 (2007).
Schleicher, E. & Friess, U. Oxidative stress, AGE, and atherosclerosis. Kidney Int. 106, 17–26 (2007).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: how are they linked. Free Radical Bio. Med. 49, 1603–1616 (2010).
Pranczk, J., Jacewicz, D., Wyrzykowski, D. & Chmurzynski, L. Platinum(II) and palladium(II) complex compounds as anti-cancer drugs. Methods of cytotoxicity determination. Curr. Pharm. Anal. 10, 2–9 (2014).
Jia, J., Ma, L., Wu, M., Zhang, L., Zhang, X., Zhai, Q., Jiang, T., Wang, Q. & Xiong, L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid. Med. Cell Longev., doi: 10.1155/2014/893516 (2014).
Rao, W., Peng, C., Zhang, L., Su, N., Wang, K., Hui, H. & Fei, Z. Homer1a attenuates glutamate-induced oxidative injury in HT-22 cells through regulation of store-operated calcium entry. Sci. Rep. 6, 33975 (2016).
Rhee, S. G., Chang, T. S., Jeong, W. & Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. cells 29, 539–549 (2010).
Żamojć, K., Zdrowowicz, M., Jacewicz, D., Wyrzykowski, D. & Chmurzyński, L. Fluorescent probes used for detection of hydrogen peroxide under biological conditions. Cr. Rev. Anal. Chem. 46, 171–200 (2016).
Holleman, M. A. F. Notice sur l’action de l’eau oxygenee sur les acides (-cetoniques et sur les dicetones 1.2. Recl. Trav. Chim. Pays-bas Belg. 23, 169–172 (1904).
Jacewicz, D., Dąbrowska, A., Banecki, B., Kolisz, I., Woźniak, M. & Chmurzyński, L. A stopped-flow study on the kinetics and mechanism of CO2 uptake by chromium(III) complexes with histamine and pyridoxamine. Transit. Metal Chem. 30, 209–216 (2005).
Jacewicz, D., Szkatuła, M., Chylewska, A., Dąbrowska, A. & Chmurzyński, L. Coordinate cis-[Cr(C2O4)(pm)(OH2)2]+ cation as molecular biosensor of pyruvates protective activity against hydrogen peroxide mediated cytotoxity. Sensors 8, 4487–4504 (2008).
Coyle, J. T. & Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Sci. 262, 689–695 (1993).
Yoon, S. W., Kang, S., Ryu, S. E. & Poo, H. Identification of tyrosine-nitrated proteins in HT22 hippocampal cells during glutamate-induced oxidative stress. Cell Prolif. 43, 584–593 (2010).
Kita, E. Model quasi-enzyme compounds of chromium(III) with vitamins B6 and histamine. Pol J Chem. 61, 361–367 (1987).
Jacewicz, D., Żamojć, K., Wyrzykowski, D. & Chmurzyński, L. Analytical methods for determination of CO and CO2 and their applicability in biological studies. Curr. Pharm. Anal. 9, 226–235 (2013).
Halliwell, B. Antioxidants in human health and disease. Ann. Rev. Nutr. 16, 33–50 (1996).
Hua Long, L. & Halliwell, B. Oxidation and generation of hydrogen peroxide by thiol compounds in commonly used cell culture media. Biochem. Biophys. Res. Commun. 286, 991–994 (2001).
Grzelak, A., Rychlik, B. & Bartosz, G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radical Bio. Med. 30, 1418–1425 (2001).
Aubin, J. E. Autofluorescence of viable cultured mammalian cells. J. Histochem. Cytochem. 27, 36–43 (1979).
Alberti, S., Parks, D. R. & Herzenberg, L. A. A single laser method for subtraction of cell auto fluorescence in flow cytometry. Cytometry 8, 114–119 (1987).
Mosiman, V. L., Patterson, B. K., Canterero, L. & Goolsby, C. L. Reducing cellular autofluorescence in flow cytometry: an in situ method. Cytometry 30, 151–156 (1997).
Steinkamp, J. A. & Stewart, C. C. Dual-laser, differential fluorescence correction method for reducing cellular background autofluorescence. Cytometry 7, 566–574 (1986).
Neumann, M. & Gabel, D. Simple method for reduction of autofluorescence in fluorescence microscopy. J. Histochem. Cytochem. 50, 437–439 (2002).
Ubezio, P. & Civoli, F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radical Bio. Med. 16, 509–516 (1994).
Rothe, G. & Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J. Leukocyte Biol. 47, 440–448 (1990).
Acknowledgements
This work was supported by National Science Centre, Poland grant numbers 2015/19/N/ST5/00276
Author information
Authors and Affiliations
Contributions
D.J. and K.S.-K. designed the study and wrote the paper. D.J., K.S.-K., J.D., A.P., D.W., A.T. and K.Ż. conducted the experiments and analysed the data. L.C. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jacewicz, D., Siedlecka-Kroplewska, K., Drzeżdżon, J. et al. Method for detection of hydrogen peroxide in HT22 cells. Sci Rep 7, 45673 (2017). https://doi.org/10.1038/srep45673
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45673
This article is cited by
-
Ultrasensitive and specific photoelectrochemical sensor for hydrogen peroxide detection based on pillar[5]arene-functionalized Au nanoparticles and MWNTs hybrid BiOBr heterojunction
Microchimica Acta (2024)
-
Novel method for rapid toxicity screening of magnetic nanoparticles
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.