Abstract
Endometriosis is a common, complex gynecologic disorder characterized by the presence of endometrial-like tissues at extrauterine sites. Elevation in protein and lipid mediators of inflammation including oxylipins and cytokines within the peritoneum characterize the inflamed pelvic region and may contribute to the survival and growth of displaced endometrial tissues. The presence of a clinically silent but molecularly detectable systemic inflammation in endometriosis has been proposed. Thus, we examined serum oxylipin and immunomodulatory protein levels in 103 women undergoing laparoscopy to evaluate systematically any involvement in systemic pathophysiological inflammation in endometriosis. Oxylipin levels were similar between women with and without endometriosis. Stratification by menstrual phase or severity did not offer any difference. Women with ovarian endometriosis had significantly lower 12-HETE relative to peritoneal endometriosis (−50.7%). Serum oxylipin levels were not associated with pre-operative pain symptoms. Changes to immunomodulatory proteins were minimal, with IL-12(p70), IL-13 and VEGF significantly lower in mild endometriotic women compared to non-endometriotic women (−39%, −54% and −76% respectively). Verification using C-reactive protein as a non-specific marker of inflammation further showed similar levels between groups. The implications of our work suggest pro-inflammatory mediators in the classes studied may have potentially limited value as circulating biomarkers for endometriosis, suggesting of potentially tenuous systemic inflammation in endometriosis.
Similar content being viewed by others
Introduction
Endometriosis is a complex gynecologic disorder characterized by the presence of endometrial-like tissues at sites outside of the uterine cavity, affecting 2–10% of women and half of women with subfertility. Pain and infertility are two prominent symptoms most commonly associated with the endometriosis and have been attributed to chronic inflammatory state of the pelvic peritoneal area with altered immunological and inflammatory milieu in the microenvironment1. This can be deduced by two main features found in the peritoneal environment–i) the increase in immune cells and ii) the elevation of pro-inflammatory immunomodulatory proteins (cytokines and chemokines) and lipid mediators such as prostaglandins in the peritoneum and peritoneal fluids of women with endometriosis2,3,4,5,6,7. There are several reports of increased circulating cytokines such as IL-6 and TNFα in women with endometriosis2,3,4,5 but discrepancies continue to pervade the literature in terms of reproducibility of the findings4,8,9. This has led to the questioning of whether endometriosis is accompanied by a clinically silent systemic inflammation10 and resulted in searches for circulating inflammatory markers which could potentially predict endometriosis.
Inflammation is biochemically modulated by oxylipins cooperating with cytokines and chemokines11. Oxylipins, collectively, includes bioactive, oxidized lipid mediators synthesized from free omega-6 polyunsaturated fatty acids (n-6 PUFA) including arachidonic acid (AA), linoleic acid (LA) and dihomo-gamma-linolenic acid (DGLA), or omega-3 polyunsaturated fatty acid (n-3 PUFA) including eicosapentenoic acid (EPA), docosahexanoic acid (DHA) and alpha-linolenic acid (ALA). Upon liberation from membrane bound phospholipids by activation of phospholipase A2 and subsequent oxidation by cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 epoxygenase (CYP450) systems, oxylipins such as prostaglandins (PG), leukotrienes (LT), thromboxanes (TBX) and hydroxyeicosatetraenoic acids (HETEs) are generated. In general, the n-6 PUFAs AA and LA are the precursors of pro-inflammatory lipid mediators while EPA and DHA derived lipid mediators resolve inflammation12. Oxylipins are known to exhibit paracrine, autocrine and increasingly endocrine effects, acting on both local and distant targets by secretion into the circulation13.
Considering the pivotal physiological and pathophysiological roles of oxylipins and immunomodulatory proteins in inflammation, we used them as assessors of systemic inflammation in endometriosis. We previously employed a targeted lipidomics approach using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) to analyze serum sphingolipids in endometriosis14 and herein, we employed a combined targeted ‘omics approach comprising LC-MS/MS and multiplex immunoassay to assess the levels of serum pro-inflammatory oxylipins, cytokines and chemokines. We report limited systemic inflammation in women with endometriosis and the results was corroborated with the non-specific inflammation marker, C-reactive protein15. Our extensive profiling here from well-defined cases and controls suggests that systemic pro-inflammatory mediators are likely to have limited diagnostic value in endometriosis. Our results inform prospective researchers in the search for biomarker discovery of endometriosis diagnosis to refrain from pro-inflammatory mediators in the circulation.
Methods
Patients and sample collection
The study population comprising of 120 patients presenting with sub-fertility in addition to a general gynaecological case-mix was recruited in KK Women’s and Children’s Hospital, Singapore. Exclusion criteria include patients who are menstruating, anovulatory, post-menopausal, on hormonal therapy for at least three months before laparoscopy or anti-inflammatory medication a day before laparoscopy and other potentially confounding diseases such as diabetes, adenomyosis or any other chronic inflammatory diseases (rheumatoid arthritis, inflammatory bowel disease, systemic sclerosis, etc). The following patients were excluded: seven menstruating patients, two with undeterminable menstrual phase, two with irregular menstrual phase, two anovulatory patients and one patient with undetermined endometriosis severity scoring. Women provided written informed consent for collection of samples and were carried out in accordance with stipulated guidelines and regulations under Centralised Institutional Research Board approval (CIRB 2010-167-D).
A diagnostic laparoscopy was performed with careful inspection of the uterus, fallopian tubes, ovaries, pouch of Douglas and the pelvic peritoneum. Presence of endometriosis is scored according to the revised American Fertility Society (rAFS) classification of endometriosis16. For the purpose of this study, we grouped rAFS Stages I and II as “mild” (EM + Mild; n = 19) and rAFS Stages III and IV as “severe” (EM + Sev; n = 38). 57 patients were diagnosed as having endometriosis (EM+) and 46 women who did not have endometriosis or have benign gynecological presentations such as uterine fibroids and benign ovarian cysts were taken as the control group (EM−). Further details on patient characteristics can be found in Supplemental Table 1. Peritoneal or ovarian endometriosis (endometrioma) were determined based on endometriosis entity grouping by Chapron et al.17,18. There were no patients with deep infiltrating endometriosis. We estimated the sample size based on a conservative lower limit of the disease prevalence at 20% in a population and an anticipated Area Under Curve (AUC) of any candidate biomarkers of 0.8, at 90% power with Type I error (false positives) fixed at 5% and Type II error (false negatives) fixed at 5%. To this, 14 Stage I/II endometriotic subjects, 14 endometriotic Stage III/IV subjects and 56 non-endometriosis women subjects was required to power the study. A value of AUC 0.5 is of no diagnostic value and 1 representing 100% sensitivity and specificity. While slightly underpowered in terms of non-endometriosis subjects, in this exploratory study the sample size serves as a useful guide for future studies.
Menstrual cycle phase was determined according to cycle history of the patients. Blood was collected in BD Vacutainer® SST II and serum prepared by spinning the tubes at 1,200×g for 10 min and the top yellowish layer transferred to a clean 15 mL tube. Subsequently, the tube was spun at 3,600×g for 10 min. The supernatant was carefully removed and transferred into 1 mL aliquots and stored at −80 °C until use.
Mass spectrometry analysis
The LC-MS/MS analysis followed a published report with some modifications19. Deuterium-labeled and non-deuterium-labeled oxylipins standards were obtained from Cayman Chemicals (MI, USA). Oxylipins were extracted from 50 μL serum by methanol–based protein precipitation and deuterated standards were added as internal standards (ISTD).
Briefly, Reversed-phase Liquid Chromatography (RPLC)-MS analysis was performed with Agilent 1290 Ultra Pressure Liquid Chromatography (UPLC, Waldbronn, Germany) coupled to an electrospray ionization with iFunnel Technology on a triple quadrupole mass spectrometer (6490 QQQ, Agilent Technologies). Chromatographic separation was achieved using HT Zorbax SB-C18 column (2.1 × 100 mm, 1.8 μm; Agilent Technologies, CA, USA) with a flow rate of 0.40 mL/min at 40 °C. The initial condition was set at 15% B, a 11 min linear gradient to 60% B was applied, followed by a 17 min gradient to 100% B which was held for 5 min, then returned to starting conditions over 0.1 min., while using Solvents A, 0.1% aqueous acetic acid and B, 50:50 v/v acetonitrile/ isopropanol. The auto-sampler was cooled at 4 °C and 10 μL of the extract was injected. Electrospray ionization was performed in negative mode with the following source parameters: drying gas (N2) temperature 200 °C with a flow of 14 L/min, nebulizer gas pressure 30 psi, sheath gas temperature 400 °C with a flow of 11 L/min, capillary voltage 3,000 V and nozzle voltage 800 V. Oxylipins were quantified in Multiple Reaction Monitoring (MRM) mode and their nomenclature as defined in Supplemental Tables 2 and 3. Data acquisition and processing were performed using MassHunter software (Agilent Technologies, CA, US).
Recoveries were evaluated by spiking defined amounts of deuterated ISTDs into aliquots of unprocessed serum and calculated by comparing peak areas from serum against mean peak areas of three equal amounts of unprocessed compounds in pure solvent. The recoveries generally ranged from 55.0% to 65.2%. For intra-batch and inter-batch precision and accuracy, the relative standard deviation (RSD) values ranged from 2.5% to 18.9% and 1.5% to 15.9%, respectively. Because chromatography separated oxylipin classes according to different retention time groups, we used the closest eluting internal standard (based on structural similarity) for relative quantitation estimates. Oxylipins were quantified by normalizing to their corresponding ISTDs as described in Supplemental Table 2. Equation 1 was used for peak area normalizations for oxylipini of samplej and ISTDk:

Multiplexed immunoassay
The Luminex xMAP multiplexing technology and the Bio-Plex® platform (Bio-Rad Laboratories, CA, USA) were used as previously employed20. The method uses 5.5 μm polystyrene beads labelled with two fluorescent dyes in different ratios, which assigns them to specific antibodies and thus allows the simultaneous measurement of 27 immunomodulatory proteins (cytokine, chemokine and growth factor) in 25 μL of serum. Serum was diluted four times prior to analysis. Data analysis of experimental data was carried out using five-parameter logistic regression modeling on the Bio-Plex system (Bio-Rad). Calibrations and validations were performed prior to analysis and on a monthly basis respectively. Measured immuno-modulators and assay parameters are reported in Supplemental Table 4.
C-reactive protein analysis
Serum CRP levels were determined via Architect C8000 (Abbott Diagnostics, Illinois, USA) according to manufacturer’s protocol.
Statistics
Data were first analyzed using D'Agostino-Pearson and Shapiro-Wilk normality tests to evaluate if they followed Gaussian distribution or not. Subsequently, the appropriate tests were used to test for statistical significance–Mann Whitney and Kruskal-Wallis test for non-Gaussian distributed data and Student’s t-test and 1-way ANOVA for Gaussian distributed data. Changes were deemed significant when p < 0.05 and % fold change >50%.
Results
Data from a total of 103 women who underwent diagnostic laparoscopy were used to assess changes of endometriosis-associated systemic inflammation. Of the 103 subjects, 46 women did not have endometriosis (designated “EM−”), 19 women with rAFS Stage I/II (designated “EM+Mild”) and 38 with Stages III/IV (designated “EM+Sev”) (Supplemental Table 1). The mean age was 34.6 ± 7.2 years (mean ± SD) with no statistical difference between groups. Chinese women form the majority (67.3%), followed by Malays (14.4%), Indians (7.7%) and women of other Southeast Asian heritage (10.6%). Women with proliferative or secretory phase were not significantly different between EM− and EM+. There was significant difference in the menstrual cycle comparing EM−, EM+Mild and EM+Sev where numbers of EM+Mild women at proliferative phase were lower (p = 0.036). There was a significant difference of the endometriosis types between EM+Mild and EM+Sev (peritoneal versus ovarian endometriosis; p < 0.0001).
Our targeted LC-MS/MS method allowed the quantification of 50 oxylipins (validated with 50 external standards) and 5 internal standards (Supplemental Tables 2 and 3) which afforded compound identity and quantification reliability and accuracy and also a targeted analysis of pro-inflammatory LA and AA-derived n-6 oxylipins. Among these 50 oxylipins, 20 were readily detectable in sera and the four most abundant oxylipins were AA, LA, 9-HODE and 13-HODE in decreasing order (mean ± standard deviation: 68.5 ± 15.9 nM, 19.3 ± 4.2 nM, 3.3 ± 2.4 nM, 3.1 ± 2.5 nM respectively). The mean concentration levels of the remaining detectable oxylipins were <1 nM. No significant difference in serum oxylipins between EM− and EM+women was found. Stratification according to rAFS stages (I and II versus III and IV) or pre-operative pain symptoms did not result in significant differences relative to EM−. Stratifying by endometriosis type (ovarian/peritoneal), women with predominant endometriomas had significantly lower serum 12-HETE relative to EM− (−50.7%; p = 0.03) (Table 1). When matched for menstrual phase (proliferative versus secretory), EM− women had significantly higher 8-HETE (54.7%; p = 0.04), 11-HETE (61.6%, p = 0.02), 15-HETE (57.65, p = 0.03) and 5-oxoETE (52.4%; p = 0.04) in the proliferative phase compared to the secretory phase (Table 2). While 14,15-DHET was statistically decreased in EM+Sev (p = 0.03), it was only 30.4% lower in the proliferative and was deemed insignificant (Table 2).
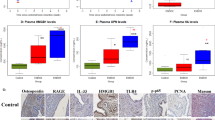
Among the 27 serum immunomodulatory proteins analyzed, 21 were detected (Table 3). The four most abundant immunomodulatory proteins were PGDF-bb, IP-10, IFNγ and IL-1rα in decreasing order (mean ± standard deviation: 7548.8 ± 4873.2 pg/mL, 1086.0 ± 501.3 pg/mL, 147.3 ± 89.2 pg/mL, 130.0 ± 70.0 pg/mL). Of the 27 factors, IL-12(p70) and IL-13 were significantly decreased by 32% and 47% between EM− and EM+ (p = 0.03 and 0.02 respectively; Table 2, Fig. 1A). In our study, IL-1rα, IL-6 and TNFα results were statistically insignificant between EM+to EM− (p = 0.90, 0.31, 0.65 respectively), EM+Mild to EM− (p = 0.24; p = 0.42) and EM+Sev to EM− (p = 0.83; p = 0.77). Interestingly, IL-12(p70), IL-13 and VEGF were significantly lower by 39%, 54% and 76% respectively in EM+Mild compared to EM− (Fig. 1B; Table 3).
Histograms of serum immuno-modulatory proteins and C-reactive protein levels in endometriosis.
(A) Cytokines, chemokines, growth factors, (B) Levels of serum IL-12(70), IL-13 and VEGF in women without endometriosis (EM−), with mild endometriosis (rAFS Stages I and II; EM+Mild) and with severe endometriosis (rAFS Stages I and II; EM+Severe). (C) Levels of serum C-reactive protein (CRP). 25th percentile, median and 75th percentile are shown by the lower, middle and upper boundaries of histogram and whiskers show minimum and maximum. *p < 0.05
Additionally, we used serum CRP as a non-specific marker of systemic inflammation. No difference in circulating CRP levels in EM−, EM+Mild and EM+Sev patients was found (p = 0.32; Fig. 1C), with median values consistent with that of healthy volunteers15. CRP levels were independent of the menstrual phases (pproliferative = 0.53 and psecretory = 0.35).
Discussion
Endometriosis is commonly associated with inflammation of the pelvic area and peritoneum. This hallmark has led to searches of inflammatory markers in the circulation which could potentially predict the presence of endometriosis and the possibility of a clinically silent systemic inflammatory state in women with endometriosis10. Our results, which covered three classes of molecules associated with systemic inflammation, namely oxylipins, immunomodulatory proteins and CRP, were largely similar with minimal differences at a level which precludes their use as diagnostic biomarkers for endometriosis. This may explain why there has been no unequivocal consensus of the circulating cytokine levels in endometriosis4,8,9.
Limited changes to systemic pro-inflammatory immunomodulatory proteins and oxylipins are consistent with reports of peripheral blood immune cell activation or cytokines in women with or without endometriosis21,22. We did not find alterations in cytokines such as IL-1Rα, IL-6, TNFα as reported by other groups2,23,24. Similar EM+oxylipin levels in the proliferative or secretory phase is congruent with others7,25. Additionally, comparing pain symptomatic and asymptomatic groups, did not yield any significant differences between the two groups. This can be plausibly reasoned by the increased expression of neurotrophic factors and nerve fibres in endometriotic lesions, eutopic endometrium and the peritoneum and consequently the frequent association of pain with the pelvic and uterine regions, rather than throughout the body26. Among the significantly different factors, with the exception of IL-13, are involved in a variety of physiologic and pathophysiologic events and not just inflammation, such as growth factor-like (IL-12(p70), VEGF and 12-HETE). IL-12(p70) mediates anti-angiogenic effects27, while 12-HETE and VEGF are a potent angiogenic factors plausibly involved in the maintenance of endometriotic lesions28,29. The significant differences in immunomodulatory proteins in EM+Mild compared to EM− are consistent with other reports5. This suggests that incremental changes in immunomodulatory proteins are likely to take place in the early phase of endometriosis development but was subverted by other unknown mechanisms later in the disease. Given the difference in our results with some reported2 and not others21,30,31 such conflicting data may be attributed to (i) the heterogeneity of the disease and/or (ii) the use of different controls in different studies: women without endometriosis but may present other benign gynecological disorders, healthy women or a combination. Alternatively, there remains a possibility that larger study cohorts may result in statistically significant findings and this study needs further verification. In addition, we did not find significant differences in serum CRP, consistent with a recent study32. Our results differed from another study which reported CRP levels to be significantly different in rAFS Stage III/IV endometriosis women in the first three days of the menstrual cycle33. Possible reasons for discrepancies include the report’s relatively smaller sample size and the temporally broader timing of sampling in our study cohort. One would note with interest that a combination of 5 proteins, permutating between plasma annexin V, VEGF, CA-125, glycodelin or sICAM-1 could predict endometriosis34–none of which are of pro-inflammatory nature.
Targeted ‘omics is a rapidly emerging bioanalytical field enabling the quantitative analysis of a large number of analytes associated with diseases35,36. We have previously demonstrated the elevated levels of serum sphingolipids in women with endometriosis, suggesting a different pathophysiological mechanism of these bioactive lipids to that of oxylipins in endometriosis14. The imbalance of n-3 and n-6 PUFAs may lead to inflammation12 and suggests that targeted profiling of n-3 PUFAs may further clarify if the role of inflammatory resolving oxylipins in endometriosis. Similarly, global ‘omics technologies including metabolomics and proteomics may further test the hypothesis of endometriosis as a systemic inflammatory disease through the potential identification of pro-inflammatory circulating metabolites or proteins. Indeed, innovation global LC-MS/MS proteomics of the serum may unravel disease-specific biomarkers37,38. Interestingly, while serum 1H-NMR and LC-MS/MS metabolomics of endometriosis patients revealed differential serum metabolites between endometriosis patients and those without, these metabolites were not considered of pro-inflammatory status39,40.
This study is the first to provide extensive profiles of pro-inflammatory protein and lipid mediators in the circulation of women with endometriosis and our results reflected a limited systemic inflammation in endometriosis. The implications of our work include the (i) pro-inflammatory mediators in the classes studied may have limited value as biomarkers for endometriosis and (ii) further ‘omics work in identifying other related markers may be warranted to definitively test the hypothesis that there is systemic inflammation in endometriosis.
Additional Information
How to cite this article: Lee, Y. H. et al. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis – a targeted ‘omics study. Sci. Rep. 6, 26117; doi: 10.1038/srep26117 (2016).
References
Herington, J. L., Bruner-Tran, K. L., Lucas, J. A. & Osteen, K. G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 7, 611–626 (2011).
Bedaiwy, M. A et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum. Reprod. 17, 426–31 (2002).
Seeber, B. et al. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil. Steril. 89, 1073–81 (2008).
Gupta, S. et al. Serum and peritoneal abnormalities in endometriosis 58, 527–551 (2006).
Pizzo, A. et al. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol. Obstet. Invest. 54, 82–7 (2002).
Karck, U., Reister, F., Schäfer, W., Zahradnik, H. P. & Breckwoldt, M. PGE2 and PGF2 alpha release by human peritoneal macrophages in endometriosis. Prostaglandins 51, 49–60 (1996).
Lousse, J.-C., Defrère, S., Colette, S., Van Langendonckt, A. & Donnez, J. Expression of eicosanoid biosynthetic and catabolic enzymes in peritoneal endometriosis. Hum. Reprod. 25, 734–41 (2010).
Bedaiwy, M. a & Falcone, T. Laboratory testing for endometriosis. Clin. Chim. Acta 340, 41–56 (2004).
May, K. E. et al. Peripheral biomarkers of endometriosis: a systematic review. Hum. Reprod. Update 16, 651–74 (2010).
Agic, A. et al. Is Endometriosis Associated with Systemic Subclinical Inflammation? Gynecol. Obstet. Invest. 62, 139–147 (2006).
Calder, P. C. Polyunsaturated fatty acids and inflammation. Prostaglandins. Leukot. Essent. Fatty Acids 75, 197–202 (2006).
Serhan, C. N., Yacoubian, S. & Yang, R. Anti-Inflammatory and Proresolving Lipid Mediators. Annu. Rev. Pathol. Mech. Dis. 3, 279–312 (2008).
Shearer, G. C. & Newman, J. W. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr. Atheroscler. Rep. 11, 403–10 (2009).
Lee, Y. H. et al. Dysregulated sphingolipid metabolism in endometriosis. J. Clin. Endocrinol. Metab. 99, E1913–21 (2014).
Pepys, M. B. & Hirschfield, G. M. C-reactive protein: a critical update. J. Clin. Invest. 111, 1805–1812 (2003).
ASRM. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 67, 817–821 (1997).
Chapron, C. et al. Smoking habits of 411 women with histologically proven endometriosis and 567 unaffected women. Fertil. Steril. 94, 2353–5 (2010).
Chapron, C. et al. Associated ovarian endometrioma is a marker for greater severity of deeply infiltrating endometriosis. Fertil. Steril. 92, 453–457 (2009).
Strassburg, K. et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal. Bioanal. Chem. 404, 1413–26 (2012).
Murakami, K. et al. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology 155, 4542–53 (2014).
Hassa, H., Tanir, H. M., Tekin, B., Kirilmaz, S. D. & Sahin Mutlu, F. Cytokine and immune cell levels in peritoneal fluid and peripheral blood of women with early- and late-staged endometriosis. Arch. Gynecol. Obstet. 279, 891–5 (2009).
Kalu, E. et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J. Obstet. Gynaecol. Res. 33, 490–5 (2007).
Kondera-Anasz, Z., Sikora, J., Mielczarek-Palacz, A. & Jońca, M. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 123, 198–203 (2005).
Cho, S. H. et al. Evaluation of serum and urinary angiogenic factors in patients with endometriosis. Am. J. Reprod. Immunol. 58, 497–504 (2007).
Liedman, R. et al. Reproductive hormones in plasma over the menstrual cycle in primary dysmenorrhea compared with healthy subjects. Gynecol. Endocrinol. 24, 508–513 (2008).
Morotti, M., Vincent, K., Brawn, J., Zondervan, K. T. & Becker, C. M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 20, 717–736 (2014).
Airoldi, I. et al. Endogenous IL-12 triggers an antiangiogenic program in melanoma cells. Proc. Natl. Acad. Sci. 104, 3996–4001 (2007).
McLaren, J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum. Reprod. Update6, 45–55.
Kim, G.-Y., Lee, J.-W., Cho, S.-H., Seo, J.-M. & Kim, J.-H. Role of the Low-Affinity Leukotriene B4 Receptor BLT2 in VEGF-Induced Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 915–920 (2009).
Vercellini, P. et al. Tumor necrosis factor in plasma and peritoneal fluid of women with and without endometriosis. Gynecol. Obstet. Invest. 36, 39–41 (1993).
Podgaec, S. et al. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum. Reprod. 22, 1373–1379 (2007).
Wessels, J. M., Kay, V. R., Leyland, N. A., Agarwal, S. K. & Foster, W. G. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil. Steril. 105, 119–128.e5 (2016).
Abrão, M. S. et al. The use of biochemical markers in the diagnosis of pelvic endometriosis. Hum. Reprod. 12, 2523–7 (1997).
Vodolazkaia, A. et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum. Reprod. 27, 2698–2711 (2012).
Nie, W. et al. Advanced mass spectrometry-based multi-omics technologies for exploring the pathogenesis of hepatocellular carcinoma. Mass Spectrom. Rev. 1–19, 10.1002/mas.21439 (2014).
Astarita, G., Kendall, A. C., Dennis, E. A. & Nicolaou, A. Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 1851, 456–468 (2015).
Al-Daghri, N. M. et al. Whole Serum 3D LC-nESI-FTMS Quantitative Proteomics Reveals Sexual Dimorphism in the Milieu Intérieur of Overweight and Obese Adults. J. Proteome Res. 13, 5094–5105 (2014).
Garbis, S. D. et al. A Novel Multidimensional Protein Identification Technology Approach Combining Protein Size Exclusion Prefractionation, Peptide Zwitterion−Ion Hydrophilic Interaction Chromatography and Nano-Ultraperformance RP Chromatography/nESI-MS 2 for the in-Depth An. Anal. Chem. 83, 708–718 (2011).
Dutta, M. et al. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol. Biosyst. 8, 3281 (2012).
Yang, B. et al. Serum metabolic profiling study of endometriosis by using wooden-tip electrospray ionization mass spectrometry. Anal. Methods 7, 6125–6132 (2015).
Acknowledgements
We thank Dr. Clement Goh and Johnson Setoh from KKH Clinical Chemistry laboratory for running the CRP analysis. This study is funded by SingHealth Foundation (SHF/FG560P/2014) and National Medical Research Foundation (NMRC/BNIG/2033/2015).
Author information
Authors and Affiliations
Contributions
The study was designed by L.Y.H. performed analysis of cytokines. C.L. and F.J.L. performed oxylipin analyses. L.Y.H. and J.C.K.Y. interpreted the data and wrote the manuscript. B.C., T.H.H. and J.C.K.Y. phenotyped the patients and obtained samples.
Ethics declarations
Competing interests
Chan J. K.Y. received salary support from the National Medical Research Council, Singapore (NMRC/CSA/043/2012).
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, Y., Cui, L., Fang, J. et al. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis – a targeted ‘omics study. Sci Rep 6, 26117 (2016). https://doi.org/10.1038/srep26117
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26117
This article is cited by
-
Dehydroepiandrosterone supplementation and the impact of follicular fluid metabolome and cytokinome profiles in poor ovarian responders
Journal of Ovarian Research (2023)
-
A Targeted Lipidomic Reveals CYP450-Derived Oxylipin Linked to the Inflammatory Response by Polycyclic Aromatic Hydrocarbon Exposure in Children
Exposure and Health (2023)
-
Metabolic Profile of Patients with Severe Endometriosis: a Prospective Experimental Study
Reproductive Sciences (2021)
-
Multiplex analysis of 40 cytokines do not allow separation between endometriosis patients and controls
Scientific Reports (2019)
-
Endometriosis foci differentiation by rapid lipid profiling using tissue spray ionization and high resolution mass spectrometry
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.