Credit: S. Fenwick / Springer Nature Limited
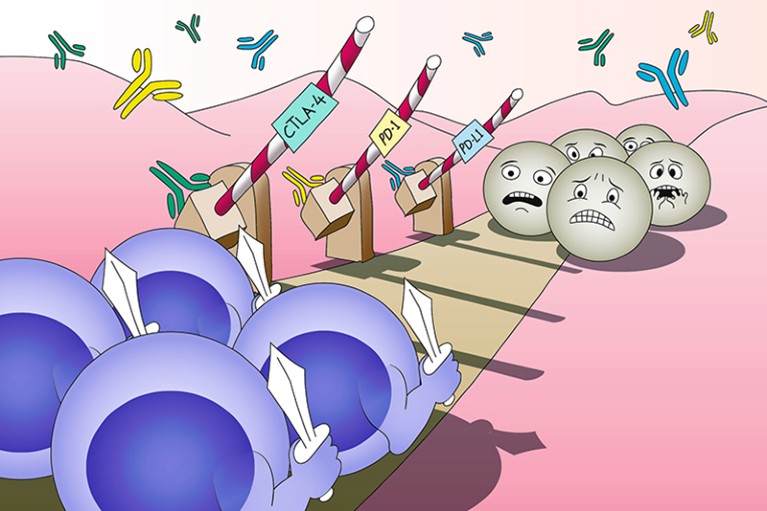
Efforts to harness the immune system for cancer therapy can be traced back over 100 years; however, cancers develop under constant immunosurveillance and therefore evolve diverse mechanisms of immunosuppression, which have proven difficult to overcome. A breakthrough that eventually led to the establishment of immunotherapy as a new pillar of cancer care was made in 1996, when James Allison and colleagues demonstrated that antagonistic antibodies targeting CTLA-4, a receptor known to suppress T cell activation, could induce tumour rejection in mice. This finding revealed the contribution of ‘immune checkpoints’ with physiological roles in self-tolerance to cancer immune evasion and simultaneously illuminated the therapeutic potential of immune-checkpoint inhibitors (ICIs). In 2003, Allison and collaborators provided clinical proof of this therapeutic concept in a first-in-human study of ipilimumab, an antibody to CTLA-4, in patients with melanoma or ovarian cancer.
Around the same time, preclinical work by Lieping Chen, Tasuku Honjo, Gordon Freeman, Arlene Sharpe and others characterized a different immune-checkpoint ligand, PD-L1, which can be found on cancer cells themselves (unlike CTLA-4 ligands, which are restricted to professional antigen-presenting cells). Furthermore, Chen and Honjo demonstrated that PD-L1 has a direct role in tumour immune evasion and can be targeted therapeutically with antibodies. Previously, Honjo had also identified PD-1, the receptor for PD-L1, on T cells. These advances spurred the development of a new class of ICIs targeting the PD-1–PD-L1 axis. In 2010, the first-in-human study of nivolumab, an antibody to PD-1, revealed durable regressions of several tumour types as well as a correlation between responsiveness and tumoural expression of PD-L1, which eventually proved to be a useful, albeit imprecise, predictive biomarker of response.
2010 witnessed a second landmark in the development of ICIs when ipilimumab became the first agent shown to prolong overall survival (OS) in patients with advanced-stage melanoma (to 10 months from a median of approximately 6 months). Accordingly, in March 2011, ipilimumab made history as the first ICI to be approved by the US FDA, thus validating the approach to immunotherapy originally proposed by Allison.
As a clearly immunogenic tumour type, melanoma became a key focus for the development of ICIs, resulting in rapid clinical advances. In 2013, objective response rates of up to 52% were achieved in a phase I trial of pembrolizumab, an antibody to PD-1. Responses were observed regardless of prior exposure to ipilimumab, hinting at distinct — and therefore potentially complementary — mechanisms of action. In fact, co-treatment with ipilimumab and nivolumab was already being tested and, in 2013, a phase I trial revealed that 53% of patients treated at the maximum doses had ≥80% tumour reductions. In 2014, pembrolizumab became the first approved PD-1 inhibitor, for ipilimumab-refractory melanoma, followed by approval of nivolumab in this setting less than 4 months later. Within a year, both pembrolizumab and the ipilimumab plus nivolumab combination had been approved in the frontline setting after superiority over ipilimumab was demonstrated in phase III trials. Remarkably, the median OS of patients treated with the combination was >5 years, emphasizing the unprecedented potential of ICIs to induce durable remission of metastatic cancers.
2015 also marked the start of a rapid expansion in approvals of PD-1 inhibitors to other tumour types, starting with non-small-cell lung cancer (NSCLC) — another malignancy in which ICIs have dramatically reduced mortality. In 2016, atezolizumab became the first anti-PD-L1 antibody to join the arsenal of ICIs, with demonstrated activity against urothelial carcinoma. ICIs have now entered the treatment armamentarium for at least 17 advanced-stage malignancies, in many cases bringing the possibility of long-term survival and perhaps even cure for a subset of patients.
ICIs are, however, not a panacea. Although generally better tolerated than other anti-neoplastic agents, ICIs come with a risk of diverse and potentially serious immune-related adverse events (irAEs). Moreover, in almost all cancers, responses are limited in frequency and duration. Initial studies revealed aberrations affecting interferon signalling (JAK1/2 mutations) and/or the antigen-presentation machinery (B2M mutation) as mechanisms of resistance. By contrast, cancers with certain mutational signatures generate large numbers of non-self epitopes (neoantigens) and are therefore particularly sensitive to ICIs. This concept was illustrated by the approval of pembrolizumab for microsatellite instability-high/mismatch-repair-deficient cancers regardless of histology — the first tissue-agnostic approval — in 2017 and for cancers with a high mutational burden in 2020.
Undoubtedly, ICIs have revolutionized cancer therapy, as reflected in the awarding of the 2018 Nobel Prize in Physiology or Medicine to Allison and Honjo. To further exploit the therapeutic potential of ICIs, these agents are being explored for earlier-stage cancers, with approved adjuvant and consolidative indications following definitive local therapy for melanoma and NSCLC, respectively. These agents are also being combined with various other treatments, resulting in approvals involving chemotherapies and anti-angiogenics. However, important issues must be addressed to further improve patient outcomes, particularly by improving on the currently suboptimal predictive biomarkers and by mitigating irAEs.

 Nature Milestones in Cancer: Interactive Timeline
Nature Milestones in Cancer: Interactive Timeline
 Improved survival with ipilimumab in patients with metastatic melanoma. (Hodi, F. S. et al., 2010)
Improved survival with ipilimumab in patients with metastatic melanoma. (Hodi, F. S. et al., 2010)