TRANSCRIPT
Izzie Clarke: 00:03
Hello and welcome to this podcast from Nature Research Custom Media and Leica Microsystems. I’m science journalist Izzie Clarke, and in this episode we explore an innovative technique that shows where proteins are located within a single cell.
The proteome is the entire set of proteins in a cell or surrounding a cell. Proteins themselves are responsible for nearly every task of cellular life, from determining the cell’s shape and its inner organization to converting nutrients and regulating a cell’s genetic information. But one challenge that researchers face is that we do not yet know the role proteins play in many kinds of diseases. Crucially, there’s a striking dependency between the proteome of a cell and where the cell is located within a tissue. And investigating this spatial context could help researchers better understand how diseases unfold within tissues and therefore help to develop new treatments.
I spoke with Florian Rosenberger from the Max Planck Institute of Biochemistry, the lead author on a recent paper in Nature Methods that explores this topic, who begins by explaining how he and his colleagues have overcome the traditional challenges in proteomics to create this exciting method.
Florian Rosenberger: 01:27
In our recent research, we’re showing that we can quantify the proteome of single cells in space. This is, first and foremost, quite an analytical challenge. The methods we had before were single-cell proteomics, where we were able to quantify a few thousand proteins of single cells that were not in space, but that would, for instance, come from cell culture, where all cells are homogeneous. And then we had a method in the lab that is called deep visual proteomics where we can get the spatial information of the proteome, but not on a single-cell level. We needed to combine a few hundred cells to get enough signal, actually, to determine the proteome of these cells. Now, in single-cell deep visual proteomics, we’re combining these two roles. We have a single-cell approach and we have this spatial approach from deep visual proteomics.
Izzie Clarke: 02:14
And you’ve been looking at the liver, specifically. So, why is it helpful to know where the proteins are within the liver, for example?
Florian Rosenberger: 02:25
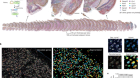
The liver is a fantastic model for the questions that we have. The question we’re targeting here is can we resolve the proteome at the level of a single cell? The liver cells, the hepatocytes, have a proteome that is very much dependent on where these cells are in the tissue. This is a phenomenon that is called liver zonation that has been known for decades and described on a couple of proteins, already years back. But we do not understand the levels of, let’s say, a couple of thousand proteins in space of these liver cells. And we know that the proteome of liver cells is very much dependent on space. At the same time, if we do now our analysis on the spatial liver proteome, we can use the already existing data to show that our methodologies are true. So we have quite a lot of biological ground truth in our data, too.
Izzie Clarke: 03:16
I know there are multiple steps, but let’s go through the process. How did you do it?
Florian Rosenberger: 03:19
We started with a frozen mouse liver in a block and took very thin sections from this liver. These sections were 10 micrometres thick. These sections are then mounted on a glass membrane slide. There is also cover of the plastic membrane. We then stain this tissue section. That means we mark particular regions that we’re interested in, and then the whole section is put into a microscope. We do a full scan of the whole section and then use an AI-based cell segmentation to outline all cells that we have in this tissue. We then pick randomly from all of these cells. In our study, we picked 450 in total, and export the precise location of these cells to laser microdissection microscope from Leica Microsystems.
Izzie Clarke: 04:07
So, essentially, you’re looking at all of these different types of cells and you’ve got this laser that can, what, just cut out each individual cell? That’s what we’re talking about here?
Florian Rosenberger: 04:17
Yes, exactly. We are identifying the outlines of the cell and we are using a laser microdissection microscope from Leica Microsystems to cut out these cells at the borders, one at a time, sorting them into a reaction container underneath. So, it’s quite a cool process. You can see the laser going around the cell and it finishes by pushing the whole, we call it shape, into a plate underneath that has 384 wells.
Izzie Clarke: 04:45
How do you know that the AI cell segmentation corresponds to the right cell type, for example?
Florian Rosenberger: 04:53
Yeah, this is a great question. Our AI-based cell segmentation outlines all cells, irrespective of whether it is an hepatocyte, for instance, or a macrophage. However, first of all, we do a filtering on cell sizes. The liver is only composed of some few cell types, and hepatocytes are by far the biggest cells, so we already do a pre-selection of this category. And then later on, what we can also do with our data is to throw out those cells where we think that they are actually not hepatocytes. Hepatocytes have a particular signature, particular proteome signature, that we already know of from other methods, which we can use for filtering our data.
Izzie Clarke: 05:32
So, once you’ve got that shape, that cell shape, what do you then do with that?
Florian Rosenberger: 05:38
Once the shapes are sorted into our reaction plate underneath the microscope, we take out the plate and we digest the proteins into peptides. This is a standard procedure in mass spectrometry. After this, we multiplex these peptides, so that means we add a mass tag to every cell in a proteome that we have in there, and combine two at a time. We also add in a reference channel that we use as a guide later on to identify the particular peptide signatures we have to look for during mass spectrometry. And these sample mixtures are then put onto our mass spectrometer. These are ultra-high sensitivity instruments that do an amazing job in detecting several thousand proteins at a time in these tiny, tiny amounts of samples, which allows us to measure on average 1,700 proteins per cell. Top numbers were 2,700 proteins per cell.
Izzie Clarke: 06:30
What would you say are some of the most important findings and how does that link to better understanding liver zonation?
Florian Rosenberger: 06:37
With this paper, we are for the first time showing that single-cell proteomics does also work in intact tissue. We are now introducing a space context to single-cell proteomics. Single-cell proteomics before has been focused very much on cultured cells, but the true biological potential and diagnostic potential, also for maybe future clinical applications, come in when we understand this space dependency of the proteome, too. One finding, of course, is that the method works, that single-cell proteomics does not just produce noise, but it actually produces a biologically meaningful data, which I think is also quite important for marketing the whole field of single-cell proteomics. Many people do amazing work there and have been doing so since years, but the community of biological researchers still has to acknowledge that single-cell proteomics has an important space in the method toolbox.
When it comes to biological insight, our data could first of all confirm a couple of spatial aspects of metabolic pathways. So for instance, we could show that the urea cycle, how it is organized in space. This has been known for decades, but we also show for other metabolic pathways how they’re organized in space and what the impact of liver zonation is. For instance, we could show that the mitochondrial compartment decreases from one region to another in a repetitive way. We could also show the precise localization of very long-chain fatty acid metabolism, and it is actually new biology that has not been shown before.
Izzie Clarke: 08:05
And what about proteome variation? Did you find anything about that as well?
Florian Rosenberger: 08:09
What we’re surprised to see is that the proteome of two neighbouring cells is actually quite different. You would think that two neighbours, they are roughly in the same environment, they will probably do about the same thing. To some extent, this still holds true, but there are still striking differences. So this underlines to us that there is such an enormous dependency on where a cell is with its proteome that it’s expressing.
Izzie Clarke: 08:34
And so just how innovative is this technology?
Florian Rosenberger: 08:35
We think it is at the forefront of the current mass spectrometry developments. Having good imaging is at the core of what we’re doing here. First of all, for good AI-based cell segmentation. But also later in our paper, we combine the image information with the proteome information and we do machine learning. And we can show that instead of the proteome of a cell, you can actually also predict the proteome of a cell. But for this, we need to have good image quality. The laser microdissector of Leica Microsystems is absolutely crucial in our experimental workflow. The laser has to be accurate enough to be able to cut out one cell at a time so that we’re not burning the full tissue, but the region where we cut with the laser is quite small. And also we need to be able to sort one cell at a time, confidently, into a reaction vessel underneath.
The LMD7 from Leica is particularly good at doing this. This is fully automated. We can, in principle, let the microscope do its job and walk away. Of course, at the current stage, we’re doing some manual monitoring, too. But I think the system has a great potential for the future when this might enter the clinic at some point, where much more hands-off work is necessary. We were also particularly surprised that, although we have so little input material, we could detect so much biology in our data. So you have to imagine that we’re taking a cell slice that is 10 micrometres thick, but the diameter of a total liver cell, an hepatocyte, is 20 to 30 micrometres.
So, at the end of the day, we are measuring here the proteome of a slice of a cell that is about a third, to a half, of a total cell. We are now quantifying up to 2,700 proteins from this one single-cell slide. But we’re also surprised to see how nicely that relates back, actually, to the spatial context of the whole tissue. And this is the power of using laser microdissection with our novel chemical labelling approaches here, and this ultra-high sensitivity mass spec pipelines.
Izzie Clarke: 10:34
How is that mass spectrometry also pushing single-cell deep visual proteomics? How important is that section to the overall process?
Florian Rosenberger: 10:45
The mass spectrometry is absolutely crucial here. So, I think only a couple of years ago, we probably couldn’t have done such an amazing job. Recent technological developments are pivotal in pushing single-cell proteomics forward. The whole field of single-cell proteomics is very much dependent on developing technologies, and I think with time we’re going to see better and better technology coming up and more exciting biology emerging from this.
Izzie Clarke: 11:06
What do you think is next for this technique? What are the possibilities with single-cell deep visual proteomics?
Florian Rosenberger: 11:14
The application of single-cell deep visual proteomics in healthy mouse liver tissue was a good benchmark. We do confirm a lot of known biology. We do uncover some new biology there. I think now is the time where this technology can grow further and to apply it also not only to healthy states, but also to diseased states. I’m particularly interested in the liver. We are bringing this to metabolic disorders of the liver where we’re also understanding the proteome, not only in the cell, but also the proteome of the extracellular matrix that surrounds the cell, for instance, in liver fibrosis or liver cirrhosis.
This, of course, can be extended also to other tissues and other diseases. And I think it will have a very important and interesting application in the context of tumours. Tumour cells, sometimes they are quite homogeneous and we do not really know how they differ in their spatial compartment. And then this method might actually help us finding this out, whether there are cells driving this or whether there is some differences between these cells and these homogeneous compartments. So, other tumours are quite heterogeneous. And then understanding the precise relation between proteome and space might give us novel insights into how these tumours work and what might be actionable protein targets to then go onto the clinic later.
Izzie Clarke: 12:31
And, so overall, how excited are you for the future of this technology and this method?
Florian Rosenberger: 12:37
I’m super excited. I think single-cell proteomics is now in a state where it start flying. So single-cell proteomics does make biological sense. This is something we’re clearly showing with our paper now. Yet it hasn’t been applied to many biological questions, yet. There are a couple of really beautiful examples already in the literature, but I think there are hundreds of thousands more to come. So I think single-cell proteomics and single-cell deep visual proteomics with the spatial component will soon enter the toolbox of biologists. And then it’s going to be just exciting to see how the technology grows, but also how we’re getting new perspectives on how cells work and how they don’t work in disease.
Izzie Clarke: 13:17
Here’s hoping. Thank you to Florian Rosenberger from the Max Planck Institute of Biochemistry. If you’d like to read more about this innovative technique, you can access the Nature Methods paper via the link doi.org/md66. Or alternatively, you can search for the paper online. It’s titled ‘Spatial single-cell mass spectrometry defines zonation of the hepatocyte proteome’. And to learn more about the technologies discussed in this episode, search for ‘LMD’, ‘DVP’ or ‘spatial biology’ on the Leica Microsystems website, www.leica-microsystems.com. I’m Izzie Clarke. Until next time.