
Using AI to help with diagnosing major depressive disorder promises to assist doctors and make diagnosis less dependent on subjective factors.© MR.Cole_Photographer/moment/Getty
Many scientists have long believed that psychiatric conditions are rooted in disordered brain networks. But it has been difficult to quantify how alterations in the brain’s functional connectivity are linked with specific disorders.
However, recent advances in brain-imaging techniques and the advent of machine-learning technologies mean that identifying distinctive brain network markers for different mental-health conditions is now within reach.
In a groundbreaking move aimed at improving the ability to diagnose mental-health conditions, XNef Inc. will apply for approval in Japan to use machine-learning software as a medical device for supporting the diagnosis of major depressive disorder (MDD)1. The trained classifier showed a similar accuracy in an independent validation dataset. In addition, diagnosis by the brain network marker was less variable than those made by clinicians.
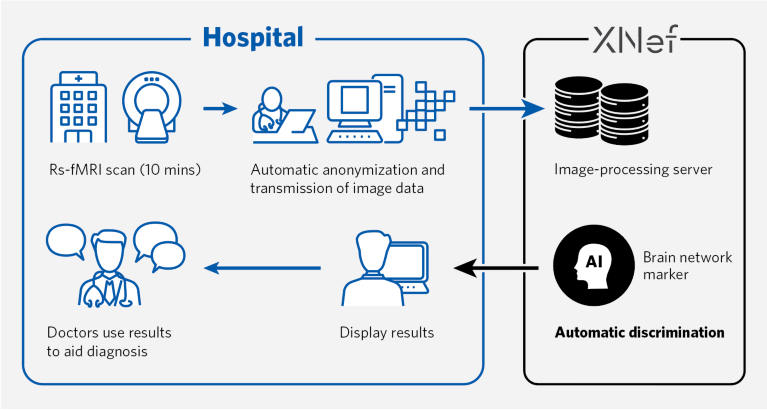
Their software employs magnetic resonance imaging (MRI) images to learn from the distribution of functional connections within the brain. It then uses these patterns in an attempt to distinguish patients from healthy controls. The MDD classifier has been trained using a discovery dataset and validated using an independent dataset.
Using this approach, XNef researchers have established brain network markers for several other neuropsychiatric disorders, including schizophrenia and autism spectrum disorder. The hope is that their machine-learning tool will one day provide an objective, biologically based method to support psychiatrists in making diagnoses.
A biological approach
MDD diagnosis currently relies on the subjective assessment of symptoms by psychiatrists. However, there is very low concordance between psychiatrists in assessing MDD. The support provided by an external, objective tool that measures a person’s brain activity would enable more balanced and accurate assessments.
“There is a critical and internationally recognized need to adopt a biological approach for mental-health diagnostics,” says psychiatrist Yuki Sakai, a leading XNef scientist and one of the company’s founders. “For example, patients tend to think of depressive symptoms as a sign of their own weakness, largely due to the lack of objective information. Using a biologically based classification technique will allow patients to externalize their symptoms as a disease of the brain network.”
The software developed by XNef hinges on resting-state functional MRI (rs-fMRI) imaging — a non-invasive procedure that takes just 10 minutes. These scans pick up connectivity patterns between specified brain nodes, and the software automatically calculates the probability of MDD.
XNef’s software (Brainalyzer) is a powerful tool for helping doctors diagnose major depressive disorder.© XNef, Inc.
Taking up the challenge
The XNef classifier is not the first to be developed using rs-fMRI data; several studies have developed machine-learning algorithms that use brain network markers to differentiate people with psychiatric disorders from healthy controls. But most of these classifiers were constructed from images taken from a small number of participants at a single imaging site.
“It’s not possible to generalize these classifiers to data obtained from other sites because of a fundamental hurdle — each MRI scanner produces slightly different images, even for the same patient,” explains Sakai.
These variations are caused by multiple factors, from differences in the MRI model to the environment in which the scanner is housed. In a multi-site, multi-participant dataset, site differences make it very difficult to identify generalizable brain network markers for individual mental-health conditions.
“To overcome this issue, we asked a group of participants to travel to multiple sites and undergo rs-fMRI,” says Sakai. “We then developed a harmonization method that smoothed out the data, removing site-specific differences to improve accuracy2.”
The researchers applied their harmonization technique to datasets drawn from every site to identify markers for specific psychiatric conditions, including their classifier for MDD. For that particular classifier, the team used a discovery dataset from more than 700 participants — including nearly 150 patients with MDD — from four sites1. They then applied classification analysis to select about 20 diagnostically useful functional connections in the brain network.
Other conditions
“It’s important to note that a specific functional connection is not related to a specific disorder,” says Sakai. “Weights are applied to the functional connections deemed most pertinent. The tool learns these, and a weighted linear summation can classify patients.”
The team has also compiled a large-scale, multi-site, multi-disorder neuroimaging database comprising rs-fMRI and structural brain images from nearly 1,000 patients and more than 1,400 healthy controls3. This database will help to identify further generalizable markers for different mental-health conditions. Initial indications suggest that the markers would be valid for different ethnicities, too1. Rapid advances in machine learning and the application of the harmonization technique are making it possible to build generalizable classifiers, Sakai notes.
Aiding drug development
Another key application for brain network markers could be drug development. Drug discovery for psychiatric disorders has stagnated in recent decades — the high costs involved and the frequent failure of randomized control studies to confirm significant differences from placebos have led to many pharmaceutical companies withdrawing from the drug-discovery process. “A key reason for this is that mental illness is a syndrome, and patients cannot easily be placed into biologically uniform groups,” says Sakai.
The brain network markers could enable researchers to stratify patients into biologically uniform subtypes. It may then be possible to conduct clinical trials focused on drugs that may work for a specific subtype. “We believe our brain network markers are a big step forward in this regard and could dramatically increase the success rate of drug discovery,” says Sakai. “It may also be possible to pre-select effective drugs for individuals based on their rs-fMRI data.” Finally, they are also developing neurofeedback techniques to improve the brain network directly.
Sakai says he looks forward to seeing this technology transform the diagnosis and treatment of psychiatric conditions. The XNef team is keen to communicate closely with psychiatrists who use their product. They acknowledge that the technology is still in its infancy and that the tool could be met with caution and scepticism.
“The brain network marker we will submit for approval is the first attempt in the world to use machine learning to assist in the diagnosis of depression. It’s impossible to predict how patients and psychiatrists will respond,” says Sakai. “It’s crucial to convey detailed information about the value of our classification tool in objective assessment, and to actively listen and act on feedback from clinicians and patients.”



 Nature Index Japan 2023
Nature Index Japan 2023