
Professor Huijun Zhao preparing an electrode for electrochemical testing and mechanistic study.
It’s hard to imagine a greener future fuel than the one under development in the labs of Huijun Zhao, Director of Griffith University’s Centre for Catalysis and Clean Energy. Zhao is working on electrocatalysts to efficiently produce hydrogen.
Sustainable ‘green’ hydrogen can be made by splitting water molecules into their component elements in an electrolyser powered by renewable electricity. The concept may be fairly clean and simple, “but technologically, water splitting is very challenging”, says Zhao. If green hydrogen is to have real-world impact, the electrocatalysts that split hydrogen from water must be made from cheap and plentiful materials that are highly effective in practical conditions.
To make hydrogen fuel, an electrocatalyst at an electrode must pluck hydrogen atoms from water molecules to combine pairs of hydrogen atoms into hydrogen molecules. But due to the inhibitory effect of the high electrical current densities passing through them, electrocatalysts can be slow at the second step. If this stage is too slow, hydrogen atoms accumulate on the electrocatalyst’s surface and choke the reaction.

A confocal Raman microscope is used to look at Griffith University's breakthrough carbon-based hydrogen electrode.
To overcome this challenge, Zhao’s team recently developed an electrocatalyst fabrication strategy in which inexpensive earth-abundant chromium sulphide and nickel sulphide electrodes are synthesized on nickel foam.
“We found with these electrodes we could overcome the inhibition effect that creates the bottleneck through a spillover process,” says Zhao. Because the team’s electrocatalysts consist of chromium sulphide and nickel sulphide active sites, the atomic hydrogen generated at the chromium sulphide active sites can be swiftly transferred to adjacent nickel sulphide sites, where hydrogen molecules are rapidly formed and released.
So far, the team’s electrocatalyst has proven stable and highly active at electrical current densities of up to 3.5 amperes, which is sufficient for industrial hydrogen production. “We think this is a real step forward for practical use,” Zhao says. Long-term stability tests under operating hydrogen production conditions are now planned.
“For sustainable future energy, hydrogen is definitely one of our best options,” says Zhao. “If you look at how hydrogen is generated, and how you can use it, the whole cycle is clean.”
And Australia has great potential to become a world leader in the green hydrogen economy, suggests Zhao. “Firstly, because Australia is one of the richest countries for solar resources, and secondly for our significant research capabilities.”
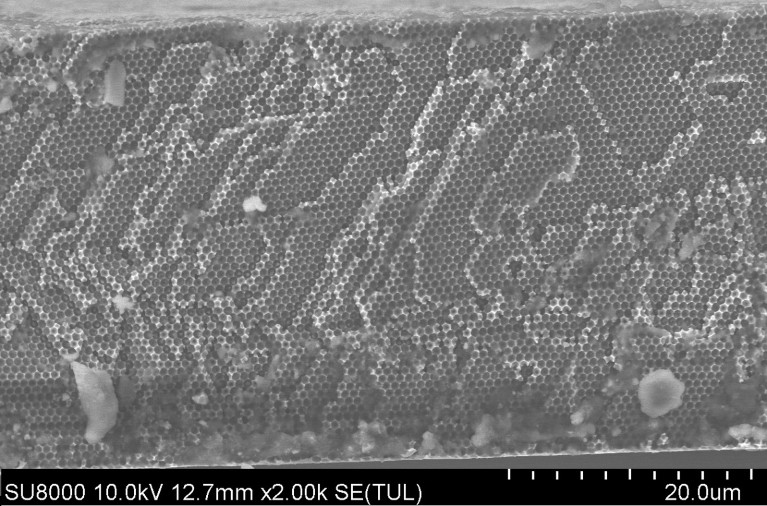
A cross-sectional scanning electron microscope image of a free-standing nickel-based electrode fabricated by Huijun Zhao's group.



 Collection: Nature Index Innovation
Collection: Nature Index Innovation