The heritage of Boehringer Ingelheim in developing medicines for respiratory diseases spans almost 100 years. Within the respiratory disease area, innovative treatments for chronic obstructive pulmonary disease (COPD), asthma, idiopathic pulmonary fibrosis, lung cancer, allergic rhinitis and infantile respiratory distress syndrome have originated from our research facilities in Germany and around the world. Specifically for COPD, our products have been on the market for more than 40 years. Looking ahead, our aim is to significantly reshape the course of the disease.
Why focus on COPD? Burden and challenges
Affecting around 300 million people globally and representing a leading cause of death worldwide1,2, COPD remains an area of significant clinical interest. The burden of the disease is expected to increase, fuelled by an ageing population2 and increases in other risk factors for COPD3,4. Although the main risk factor is cigarette smoking, air pollution and other environmental factors also play a part3,4. The use of e-cigarettes (vaping) is also rising and, although its impact has not yet been elucidated, vaping may cause lung damage and has been associated with reports of lung disease3.
COPD is a complex and heterogeneous incurable disease that can vary greatly in how it presents from patient to patient, even in those with a similar degree of airflow limitation5. It comes in several forms, one of which is emphysema, which causes progressive damage to the air sacs or alveoli of the lungs. Typical symptoms associated with underlying lung damage include breathlessness (particularly during activity), a persistent cough and/or wheezing, and frequent bronchitis. Despite treatment, the breathing problems tend to get worse over time, and patients may become increasingly less active.
The early stages of the disease are largely asymptomatic; as a result, COPD is often diagnosed late, with more than three-quarters of individuals already having moderate or severe/very severe airflow obstruction at the time of diagnosis6. The small airways, often referred to as the silent zone of the lungs, are regarded as one of the first sites where COPD develops. The surface area of the small airways is vast (Fig. 1), which means it is possible for a large proportion of functional lung tissue to be destroyed before the disease becomes symptomatic.
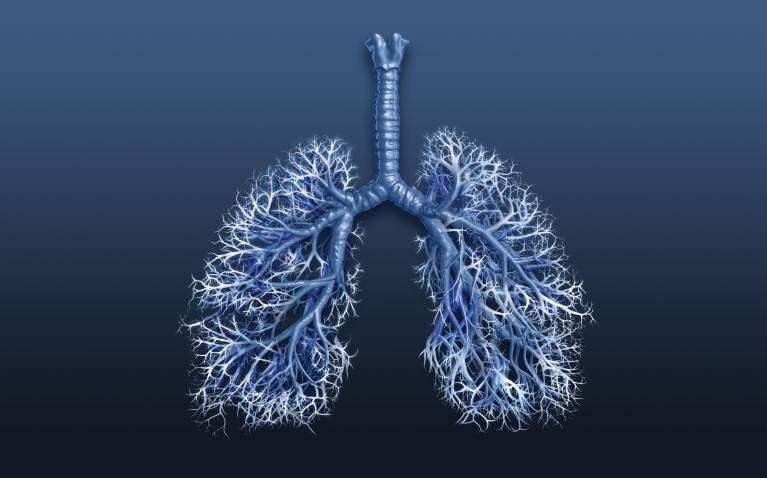
Figure 1. The small airways: The silent zone of the lungs.
Under-reporting of symptoms is common in COPD3. Many people without significant spirometric evidence of COPD still suffer from respiratory symptoms such as breathlessness, cough and sputum production, and are at increased risk of death and disease progression. Nevertheless, these people are not on the physicians’ radars according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria3,7. This has prompted the proposal of new, broader criteria for diagnosing COPD, encompassing environmental exposure, clinical symptoms and computed tomography (CT) imaging, as well as spirometry findings7. Such an approach could potentially allow us to target the disease ahead of irreparable damage.
The goals of COPD treatment include reducing symptoms, preventing and treating exacerbations, reducing deaths related to the disease3, and changing the underlying nature of the disease (otherwise known as ‘disease modification’). Current therapies provide symptomatic relief and can reduce the risk or severity of exacerbations, but no pharmacological treatments are available yet to prevent the progression of lung destruction.
The role of bronchodilation
Bronchodilator therapy forms the mainstay of treatment for people with COPD. Bronchodilators work by altering the airways’ smooth muscle tone, leading to widening of the airways, which, in turn, improves expiratory flow3. Since obstruction of the airways and associated symptoms (in particular, breathlessness) are primary concerns for most patients with COPD, the development of effective and well-tolerated bronchodilators has long been an important goal in COPD therapy.
As an alternative to increasing the dose of one bronchodilator, global guidance from GOLD recommends combining two bronchodilators with complementary mechanisms of action to increase the impact of bronchodilation3. Indeed, combining a long-acting muscarinic antagonist (LAMA) with a long-acting β2-agonist (LABA) does improve bronchodilation and patient outcomes compared with a LAMA or LABA alone3.
We have an established history in the field of bronchodilators for use as maintenance therapy in people with COPD (Fig. 2). This includes the LAMA Spiriva® (tiotropium; first available in 2002), delivered using the HandiHaler® and then through the Respimat® Soft Mist™ Inhaler, and the LABA Striverdi® (olodaterol; first available in 2014), delivered using the Respimat®. More recently, in 2015, the fixed-dose dual LAMA/LABA combination therapy Spiolto®/Stiolto® (tiotropium/olodaterol), delivered using the Respimat®, was approved for use in COPD and is currently available in 87 countries.
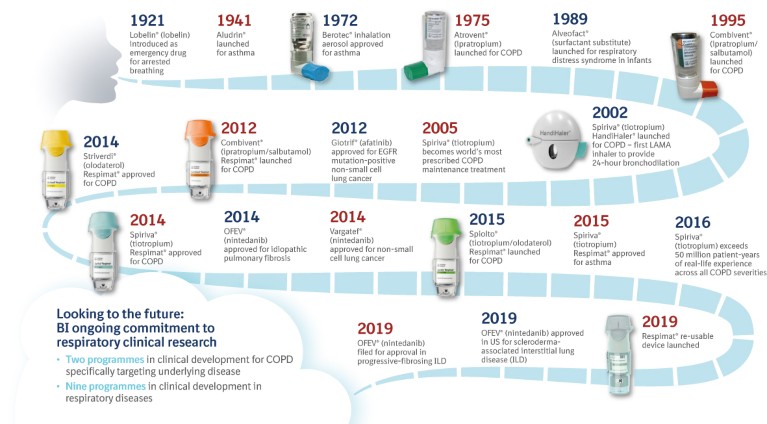
Figure 2. Boehringer Ingelheim: A heritage in respiratory diseases.
The combination of tiotropium/olodaterol increases lung function, improves health-related quality of life and reduces breathlessness compared with tiotropium or olodaterol alone, with no observed difference in tolerability8,9. Tiotropium/olodaterol also increases inspiratory capacity and exercise endurance10 versus placebo and single bronchodilator therapies. This is of particular importance because many patients can fall into a vicious cycle of activity avoidance, muscle deconditioning and increased breathlessness, leading to further physical inactivity to the detriment of their health-related quality of life. The use of bronchodilator therapies may allow individuals with COPD to maintain or increase their activity levels and improve their long-term prognosis.
The case for early action
Even in people with mild COPD, symptoms such as breathlessness are often apparent and are generally associated with exercise intolerance and restriction of physical activity. However, those who are affected may not seek medical help until symptoms are persistent and significant respiratory impairment is present.
For most patients, GOLD recommends initial pharmacological treatment with a single bronchodilator therapy, whereas dual LAMA/LABA therapy is recommended for more symptomatic patients3. However, there is a growing case for maximising bronchodilation as early as possible in the disease course. We have analysed data from our large clinical trial programme to evaluate the merits of dual bronchodilation. These results show the benefits of dual therapy with tiotropium/olodaterol in a wide variety of patient types, including treatment-naïve patients and patients with different degrees of symptoms and COPD severity9. Triple therapy with LAMA/LABA/inhaled corticosteroids also has an important place – escalation to triple therapy may be necessary for patients who develop further exacerbations on dual bronchodilation, especially among patients with higher eosinophil counts3.
Innovation in inhaler development
How the drug treatments are delivered to their site of action in the lungs is hugely important. Historically, we were responsible for launching the first commercially available metered-dose inhaler (MDI) in Europe and the United States. Devices we have developed for use in people with COPD include the Atrovent® and Combivent® unit dose vials (for use in nebulisers), the HandiHaler® dry powder inhaler (DPI), the Atrovent® and Berodual® pressurised MDIs (pMDIs) and, most recently, the Respimat® Soft Mist™ Inhaler, which is available in both disposable and re-usable formats (Fig. 2).
Respimat® Soft Mist™ Inhaler: a feat of engineering
Prior to the development of Respimat®, we were faced with a paradox. Although a large number of patients are prescribed inhalers such as DPIs, which require active inhalation for successful drug delivery, many are unable to inhale strongly enough or do not consistently perform a forceful inhalation manoeuvre, meaning that they do not use their inhalers effectively11,12. pMDIs, on the other hand, have different challenges, only needing a relatively slow and deep inhalation, but requiring coordination between activating the device and breathing in to ensure that the drug particles reach the lung efficiently11,12. Ineffective use of both DPIs and pMDIs can result in deposition of drug particles in the throat and mouth12,13. It is essential, therefore, that an appropriate inhaler is matched to each patient, with device choice being important, as is the drug selected for treatment. In fact, GOLD recommends that patients who are unable to master their inhaler may need to consider a change in delivery device3.
In response to this conundrum, our in-house engineers developed the Respimat® Soft Mist™ Inhaler (Fig. 3). This innovative new-generation propellant-free inhaler is driven by the desire to solve patients’ problems with existing inhalers while maintaining a low carbon footprint12,14. A key technical breakthrough in the development of the Respimat® was the Uniblock – the nozzle system of the inhaler – that combines filters and nozzles made of silicone and glass, inclined at a precise angle, so that two fine jets of liquid converge at a carefully controlled angle to create a slow-moving aerosol, from which the term ‘soft mist’ is derived, for optimised drug delivery12. Drug solution is forced through this system using mechanical energy from a unique spring-loading mechanism to generate a fine aerosol of inhalable fine droplets12. Respimat® generates a slow-moving, long-lasting ‘soft mist’ of drug, which can make it easier to inhale, and help provide a higher deposition of drug to the lungs compared with DPIs or pMDIs13,15.
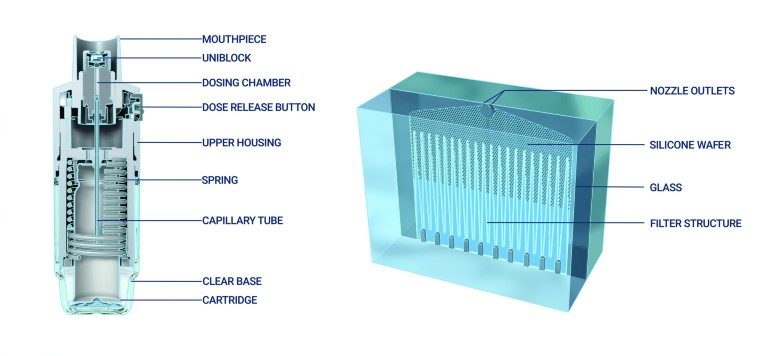
Figure 3. A cross-sectional image of the Respimat device12. This work is licensed under a Creative Commons Attribution 4.0International License (http://creativecommons.org/licenses/by-nc/4.0/).
The use of the Respimat® inhaler also has good implications for treating small airways disease (SAD), an early site of lung deterioration in COPD13,16. Believed to be present in around three-quarters of patients with COPD, SAD is difficult to diagnose and assess due to the inaccessibility of the small airways16,17. Given the overwhelming evidence for the importance of SAD in the development of COPD, the use of inhaled treatments that optimise delivery to the small airways is essential.
Importantly, Respimat® produces aerosol droplets of an appropriate size to ensure drug delivery throughout the lungs, including the small airways, without loss of small droplets during exhalation13. In vivo scintigraphy and in vitro models, based on CT imaging and computational fluid dynamics, have been used to demonstrate the effective deep lung deposition with the Respimat® inhaler; when compared with a range of different DPIs, Respimat® was shown to cause the lowest deposition of aerosol particles in the throat and the highest deposition in all regions of the lungs13.
Carbon footprint and environmental benefits of the Respimat® inhaler
Protecting the environment, conserving natural resources and promoting environmental awareness are principles that are highly valued at Boehringer Ingelheim.
A vast number of people use inhalers; in fact, in the United Kingdom (UK) an estimated 1.2 million people are living with diagnosed COPD (most of whom will be prescribed inhaled therapy), while 5.4 million people are currently receiving treatment, again mostly inhaled therapy, for asthma. Since MDIs account for nearly 4% of UK National Health Service (NHS) greenhouse gas emissions, use of hydrofluoroalkane propellants in inhalers is coming under increasing scrutiny. The NHS has pledged to set a target for at least 50% of prescribed inhalers to have low global warming potential values by 202214. Respimat® uses mechanical energy and is propellant-free, making it an environmentally friendly option. In fact, the product carbon footprint of the original disposable Respimat® inhaler is approximately 20 times smaller than hydrofluoroalkane pMDI products and similar to that of DPIs14.
Further reduction in environmental impact has been provided by the novel second-generation Respimat® re-usable inhaler, which was first launched in 2019. Prompted by feedback from patients and physicians, this was an evolution of the disposable Respimat®, and an example of our commitment to patient-centric and environmentally friendly inhaler design. The new re-usable inhaler can be used with up to six cartridges, providing increased convenience for patients while also improving ease of use15. Crucially, over the six-month lifespan of the re-usable Respimat®, there is a threefold reduction in its carbon footprint compared with the disposable Respimat® inhaler, representing a considerable contribution to sustainability14. In recognition of this contribution, the Respimat® re-usable was the winner of the 2020 Pharmapack Eco-Design Award. The Pharmapack awards celebrate the latest innovations from packaging companies within the drugs, medical devices, health products and veterinary drugs sectors.
Future directions: the role of precision medicine in COPD
We are building upon our strong heritage in bronchodilator and inhaler development with ongoing clinical development programmes aimed at improving the outlook for people with COPD. COPD is a heterogeneous and progressive disease. Both COPD itself and emphysema – a major cause of airflow limitation in COPD – are associated with reduced and declining lung function. Although current pharmacological treatment options can improve lung function, ultimately, they do little to prevent or reverse the decline in forced expiratory volume in 1 second – the most commonly used marker of disease severity and progression in COPD – over time. A more in-depth understanding of disease progression is needed to target future therapies.
Biomarkers, including those associated with emphysema and SAD, may assist in characterising patients, both in terms of response to therapy and also in predicting and monitoring the course of disease. They may also help to identify mechanisms responsible for lung destruction in COPD, which in turn could identify new treatment targets that bronchodilators – although a mainstay of therapy – do not directly impact.
To supplement findings from ongoing studies such as COPDGene® (copdgene.org), the British Lung Foundation Early COPD Study (imperial.ac.uk/blf-early-copd-partnership), ECLIPSE (eclipse-copd.com), SPIROMICS (spiromics.org), COSYCONET (www-mhh.asconet.net) and CanCOLD (cancold.ca), we are carrying out the FOOTPRINTS® study (NCT02719184), which hopes to identify biomarkers of COPD disease progression – particularly emphysema or lung function loss progression – over a three-year period (Fig. 4).
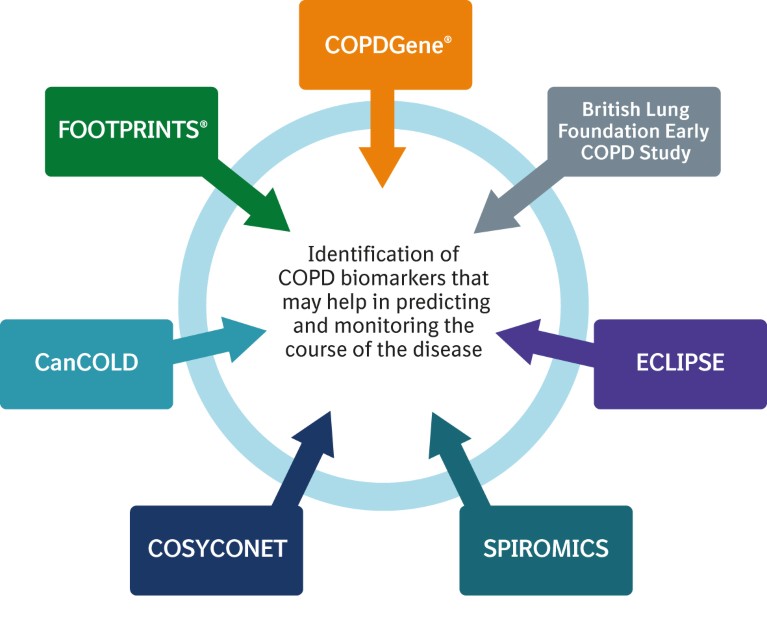
Figure 4. Key studies investigating biomarkers in chronic obstructive pulmonary disease (COPD).
FOOTPRINTS® is assessing a range of biomarkers in blood and other biofluids in former smokers with COPD versus otherwise healthy former smokers. These include biomarkers relating to protease activity, extracellular matrix biomarkers and inflammatory biomarkers. Airway wall thickness, emphysema and air trapping are being assessed using CT.
By correlating patient characteristics, biomarkers of inflammation and tissue integrity, imaging findings and lung function, the progression of lung destruction and emphysema progression may be better understood. This in turn may support the development of future treatments to slow or halt disease progression in COPD and identify those patients who are at greatest risk for disease progression. FOOTPRINTS® is also aiming to identify different COPD patient types and their relative risks of disease progression. This could include the identification of ‘rapid progressors’, who have greater unmet need.
Future directions: modifying the course of the disease
In line with our clinical research in precision medicine, we are also developing innovative treatments that can further improve the lives of people with COPD. We are researching new molecules with the potential to slow lung destruction and therefore modify the course of the disease, with the ultimate goal of developing a maintenance treatment that slows emphysema progression. By targeting patients with preserved ratio-impaired spirometry (a high-risk group for developing COPD), it might be possible in the future to even prevent or reverse lung damage7.
Recently, attention has focused on the link between COPD-related inflammation and tissue damage, and the imbalance between a type of enzymes called serine proteinases and their inhibitors (Fig. 5). Several serine proteases, including neutrophil elastase, cathepsin G and proteinase-3, are implicated in the destruction of alveolar tissue18, and are therefore potential targets for treatment.
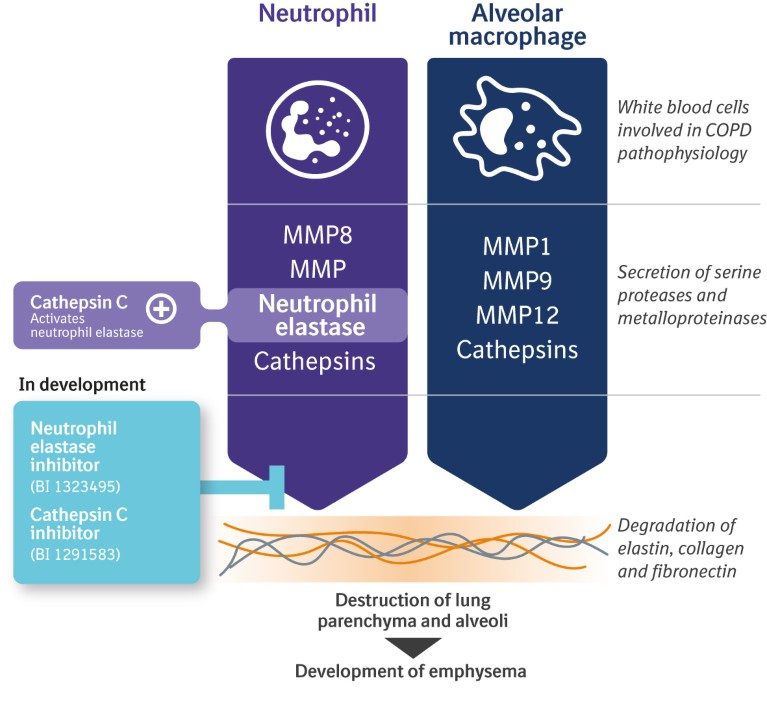
Figure 5. Potential targets to modify the course of chronic obstructive pulmonary disease (COPD).
Although these mechanisms have been investigated previously19,20, the degree of inhibition on neutrophil elastase activity in patients was only moderate. We are currently evaluating neutrophil elastase and cathepsin C inhibition in preclinical and clinical studies to explore the exciting potential for these molecules in COPD and other respiratory diseases, with the hope of moving beyond improving lung function into modifying the course of the disease.
Although there is much to celebrate regarding our heritage in respiratory diseases – spanning almost 100 years of innovation in drug and device development – there is continuing medical need for therapies that can change the underlying nature of the disease. Given that currently available treatments focus on symptom control and risk reduction, we need to maximise the effectiveness of those therapies by focusing on early and accurate diagnosis, optimising bronchodilation and tailoring the treatment selection to the patient needs. This in turn could help to optimally manage symptoms in order to maintain and improve patient quality of life and reduce the risk of experiencing exacerbations. It is also important to match the inhaler selection to patients’ ability and preference, including the use of innovative inhalers such as the Respimat® and Respimat® re-usable, to help optimise management of COPD. Meanwhile, our ongoing research and clinical programmes aim to target the power of precision medicine. By enhancing our knowledge of COPD-specific biomarkers, and continuing to assess innovative new treatment targets, we hope to meet our goal of modifying the course of the disease.
Only by doing this can we improve the outlook for people with COPD.

