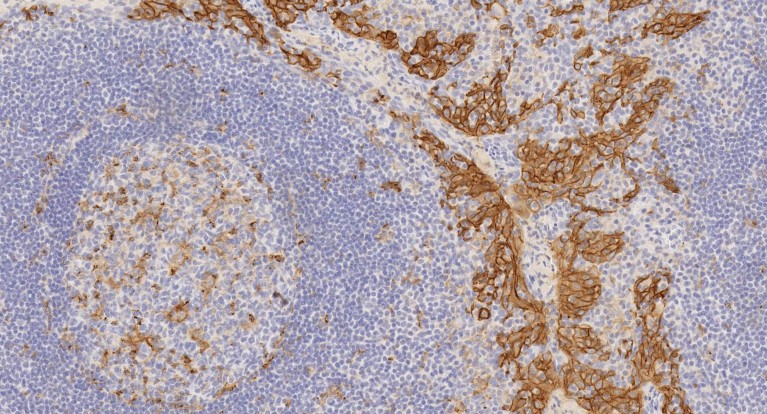
Immuno-histochemical staining of a section of tonsil using anti-PD-L1 antibody ab228415 [clone 73-10] reveals the presence of the protein (brown staining).
Cancer treatment has undergone a revolution in the past few years. What’s special about these new drugs is that they do not target tumours directly, but instead unlock the brakes that cancer puts on the immune system, allowing the body’s own defences to get to work. The keys to the lock are immune checkpoints such as programmed cell-death ligand 1 (PD-L1), and its receptor PD-1; discovering this system earned Nobel Prizes for James Allison, at the University of Texas MD Anderson Cancer Center in Houston, and Tasuku Honjo at Kyoto University in Japan,in 2018.
To date, six anti-PD-1/PD-L1 drugs have been approved by the US Food and Drug Administration (FDA) to treat 14 types of cancer and there are more than 2,000 clinical trials evaluating PD-1/PD-L1 checkpoint inhibitors underway1.
Inhibitors of PD-1 or PD-L1 have been shown to be effective against several cancer types — even when administered after conventional therapy has failed. In many cases, for instance non-small-cell lung cancer, these checkpoint inhibitors have given months or even years of life back to patients who were out of treatment options2. What’s more, they are better tolerated than conventional chemotherapies3. But not all patients respond to them, and some develop resistance4. Understanding the factors that determine response to PD-L1 blockade is essential to broadening the application of cancer immunotherapies, enhancing their efficacy, and targeting treatment appropriately. These goals are driving extensive research efforts into basic PD-1/PD-L1 biology.
A matter of expression
“For drugs against PD-1/PD-L1 to be effective, the tumours have to express some level of these targets,” says Caroline Robert, head of the Dermatology Unit, Institut Gustave Roussy, France. Several studies have shown that detection of PD-L1 protein on tumour cells before treatment correlates with a positive response to checkpoint inhibitors. However, some patients who seem to be PD-L1-negative still benefit from this therapy5, highlighting the need for more sensitive approaches to detecting the protein.
“Knowledge of the mechanisms of PD-L1 expression and the immunological consequences of blocking PD-1 signalling might help us predict and improve patients’ response,” Robert explains. One of the problems in determining PD-L1 expression is that it is dynamic; expression changes over time6 and is influenced by cells, such as antigen-presenting cells — which also express PD-L1, in the surrounding microenvironment (see ‘How tumour cells hide’).
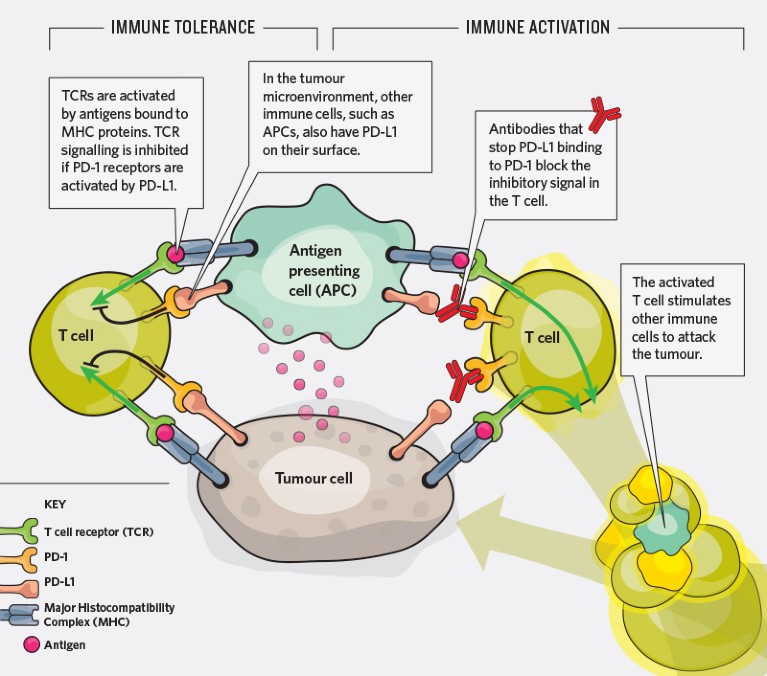
How tumour cells hide. Some cancer cells carry PD-L1, which is normally expressed on immune cells. PD-L1 promotes tumour tolerence by inhibiting signals from the T cell receptors (TCRs). Using antibodies to block PD-L1 prevents the inhibitory signals so that TCRs can initiate an antitumour immune response.
An essential task is to reliably and accurately identify these proteins on different cells in the tumour tissue, then interrogate the microenvironment for patterns of expression that could inform treatment strategies. Researchers use antibody-based detection methods such as immunohistochemistry (IHC) to explore the spatial distribution and levels of PD-L1 on both tumour and immune cells. There are several monoclonal antibodies available for IHC detection of human PD-L1, which vary in the specific part of the protein they target, the animal species they have been raised in, and in their binding affinity and specificity. When compared in the same applications, however, the antibodies produce different results7.
Houssein Abdul Sater, a research physician at the National Cancer Institute in Bethesda, Maryland, is collaborating with global life sciences company Abcam to optimize the detection of PD-L1 in particular tumour tissues prepared for immunostaining. “We are examining a full panel of anti-PD-L1 antibodies to determine how they perform in different cancer tissues in order to establish a more robust method for detecting PD-L1,” he says.
Sater and his team are finding that applying different anti-PD-L1 antibody clones to the same type of tumour tissue can produce different staining patterns. “Non-specificity can be a big problem,” he says. “If the anti-PD-L1 antibody binds non-specifically to other cells in the tumour microenvironment, it is difficult to determine the true PD-L1 status of the tumour.” Their results, due to be published later this year, will be a helpful resource to improve the assessment of PD-L1 in cancer tissue.
It is still a mystery why the antibodies work differently in different tissues, but one that Sater is keen to solve. “Given the implications of PD-L1 status for treatment management, it is important to characterize the antibodies and ensure that the best performing one is used in each tissue.”
Making the right choice
These emerging differences among validated anti-PD-L1 antibodies are challenging some long-held beliefs, says John Baker, Abcam’s Senior Vice President, Product Portfolio & Innovation, in Cambridge, UK. “PD-L1 antibodies have broadened the often binary thinking about antibodies — that is, do they bind specifically to their target or not.” Researchers now, says Baker, should also think about “which domains or epitopes are targeted, and the binding kinetics”. Whereas normally, having one really good antibody would be enough to study a given target, the case of PD-L1 suggests that the situation is much more complex when targeting a clinically important molecule.
Research into these differences has been hampered by the lack of availability of anti PD-L1 antibodies developed for diagnostic use. However, the situation is changing. “We are witnessing a shift in mentality,” says Baker. ”Pharma and diagnostic companies are recognizing the value of releasing research-use only (RUO) formulations of their proprietary clones into the research space, so they can be further validated by basic research.”
This is a win–win situation. As more data become available on the performance of these antibodies in different platforms, transparency increases — alongside researchers’ confidence in the reagents. In addition, basic and translational research on PD-L1 accelerates. Baker sees a virtuous circle: “Many antibody clones start in the clinic and then, following use in basic research, some are picked up for the development of new translational and clinical applications.”
Abcam offers a comprehensive range of recombinant rabbit monoclonal antibodies against PD-L1 to help researchers decide which is best suited to their needs. Three of them are RUO versions of anti-PD-L1 RabMAb® antibodies currently employed in the clinical setting (73-10, 28-8, SP142), which have been co-developed with pharmaceutical and diagnostic companies using Abcam’s technology. These antibodies enable researchers to study PD-L1 expression and function with well-understood and characterized clones.
Future developments
The dynamic nature of PD-L1 expression during cancer progression and treatment is difficult to monitor. Robert suggests that using more than one biomarker of tumour growth could improve assessments of treatment response and prognosis. “Composite biomarkers that integrate PD-L1 as well as additional markers or, better still, dynamic biomarkers to visualize the interaction between PD-1 and PD-L1 would help overcome some of the current challenges,” she says.
By working with academic and industry partners, Abcam is developing customizable immunoassays that simultaneously analyse multiple proteins. “We are building a knowledge network that is helping to establish standards and best practice in basic PD-L1 research, as well as driving the development of reagents to reliably and reproducibly detect disease biomarkers,” says Baker.
Given the rate at which cancer immunotherapies are entering clinical trials, researchers urgently need to optimize such tools to better understand how PD-L1 biology influences antibody efficacy.


