Scarring of the liver due to long-term liver damage, known as cirrhosis, can be life-threatening. Many diseases and conditions, such as infection with the hepatitis virus or chronic alcoholism, can cause cirrhosis. Because the liver is very good at repairing itself, patients often show no signs or symptoms of disease until the organ’s function is severely compromised. Researchers at the Translational Research Center for Medical Innovation in Kobe, Japan, are helping to develop life-changing therapies that can reverse what was previously thought to be irreversible liver scarring.
Currently, no cure exists for cirrhosis; the main aim of treatments is to slow down the progression of disease. If the liver is badly damaged, a liver transplant may be the only treatment option. The risk of complications associated with transplant surgery, with the drugs required to prevent organ rejection and of disease recurrence, are high.
The first stage of cirrhosis, known as fibrosis, is characterized by excessive accumulation of extracellular matrix proteins leading to a gradual loss of liver-cell function. Research into the mechanisms of fibrosis has identified several molecular targets for development of antifibrotic drugs. So far, no drug has been approved for clinical use.
However, recent clinical trials involving stem cells are offering new hope to patients. Based on an earlier study in mice1, Isao Sakaida at Yamaguchi University Graduate School of Medicine, Japan, was the first to show that human patients’ own bone marrow cells infused through a peripheral vein promoted liver regeneration, improved liver function and reduced fibrosis2. Bone marrow cells naturally migrated to injured livers and stimulated the differentiation of hepatic progenitor cells into functional liver cells without causing adverse effects. In some patients, the improvement in liver function was sustained for more than five years3.
In a mouse model of cirrhosis, Sakaida and colleagues also found that human bone marrow-derived cells induced the expression of enzymes involved in the breakdown of extracellular matrix proteins (matrix metalloproteinases; MMP).
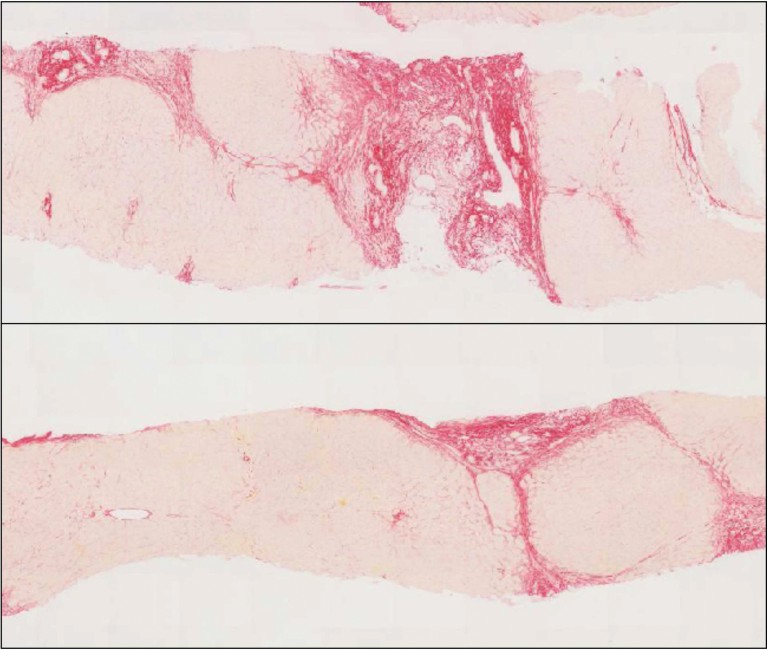
Figure 1: Liver histology before and after PRI-724 treatment (10mg/m2/day).Collagen is stained with sirius red. Image courtesy of Kiminori Kimura.
Interestingly, a similar mechanism of action seems to be behind a small-molecule drug candidate that has recently been shown to improve liver function in mice with hepatitis C virus-induced liver fibrosis. Kiminori Kimura at Tokyo Metropolitan Komagome Hospital and his team showed that a weekly injection for six weeks of PRI-724, a Wnt-signalling pathway inhibitor that interferes with beta-catenin-mediated gene transcription, significantly ameliorated liver fibrosis in a dose-dependent manner4. PRI-724 promoted extracellular matrix degradation by increasing the levels of MMP-8 and MMP-9 in the liver and inhibiting hepatic stellate cell activation. Although the mechanistic details are still unclear, the observed recruitment of anti-inflammatory cells into the liver is likely to contribute to the antifibrotic effects of PRI-724.
With funding from the Japan Agency for Medical Research and Development, a singlecentre, open-label phase 1 trial in hepatitis C cirrhosis patients has been carried out to determine the safety, tolerability and efficacy of PRI-724. The drug was well tolerated at low doses and encouraging antifibrotic activity was observed after 12 weeks of treatment (Fig.1)5. The ongoing phase 1 / 2a clinical trial (NCT03620474) to evaluate the effect of PRI-724 for hepatitis C and hepatitis B cirrhosis may accelerate the development of future therapeutic drugs.
ABOUT TRI
The Translational Research Center for Medical Innovation (TRI), formerly known as Translational Research Informatics Center, was founded in 2002 by the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Kobe city to promote academia-originated medical innovation. TRI established the Academic Research Organization (ARO) network in 2013, which is transforming into an Asian ARO network in conjunction with Korea, Taiwan and Singapore. We plan to expand the network globally to Europe and the United States. Our aim is to develop an infrastructure to support the launch of global clinical trials of academia-originated projects and to obtain regulatory approval worldwide.





