Nanomedicine has opened new horizons for clinicians, making possible novel medical tools and therapies. But how do you safely and accurately work with tools that are 140 times smaller than a red blood cell? How can you see what you’re doing when working at scales smaller than the wavelength of visible light?

Toshio Hirano, President of QST, is an immunologist who discovered interleukin-6.
That’s where imaging is essential. Multiple technologies must be dovetailed to create a seamless ‘picture’ of what is happening inside the body at the nanoscale. This is what researchers are doing at the National Institutes for Quantum and Radiological Science and Technology (QST), the core research centre for quantum science and technology in Japan.
We’re developing new in vivo imaging technologies that can recognize disease, evaluate the benefits of therapy, and discover new diagnostic biomarkers for pre-clinical applications,” says Dr Ichio Aoki of the National Institute of Radiological Sciences (NIRS) in Chiba, a major QST node.

Dr Ichio Aoki with a pre-clinical 7-Tesla MRI system.
If researchers are to understand dynamic biological processes in the body — including enzyme and protein activity, biodistribution and the progression and treatment of diseases — and do so in repeatable, longitudinal studies, accurate imaging of patients in vivo is vital. But it’s a lot harder than it sounds, especially with intractable cancers.
Take the use of magnetic resonance imaging: it provides good soft-tissue contrast, but conventional MRI lacks the sensitivity to identify cancerous cells. Positron emission tomography (PET) has much better sensitivity for detecting cancer but requires radioactive chemicals — meaning that cancer patients can be over-exposed to radiation during longitudinal studies.
Aoki’s Quantum-state Controlled MRI Group at QST has been working on ways to make MRI better at imaging tumours by developing nanoparticle contrast agents that respond and amplify the signal for tumour-related factors, such as pH and oxidation potential.
One of these, known as an MnCaP-nanomicelle, is comprised of calcium phosphate doped with manganese cation (Mn2+) nanoparticles and encased in a polyethylene glycol (PEG) shell. This pH-activated nanoparticle has shown excellent signal amplification for the imaging of tumour malignancy via MRI.
The nanoparticle-amplified MRI concept was tested with a common cancer model: colon-26 carcinoma tumour-bearing mice. Before the application of the MnCaP-nanomicelle contrast agent, three-dimensional MRI scans of the solid tumours showed only blood vessels. After adding MnCaP-nanomicelle, however, the tumour was easily distinguishable and the contrast signal remained strong for some time.
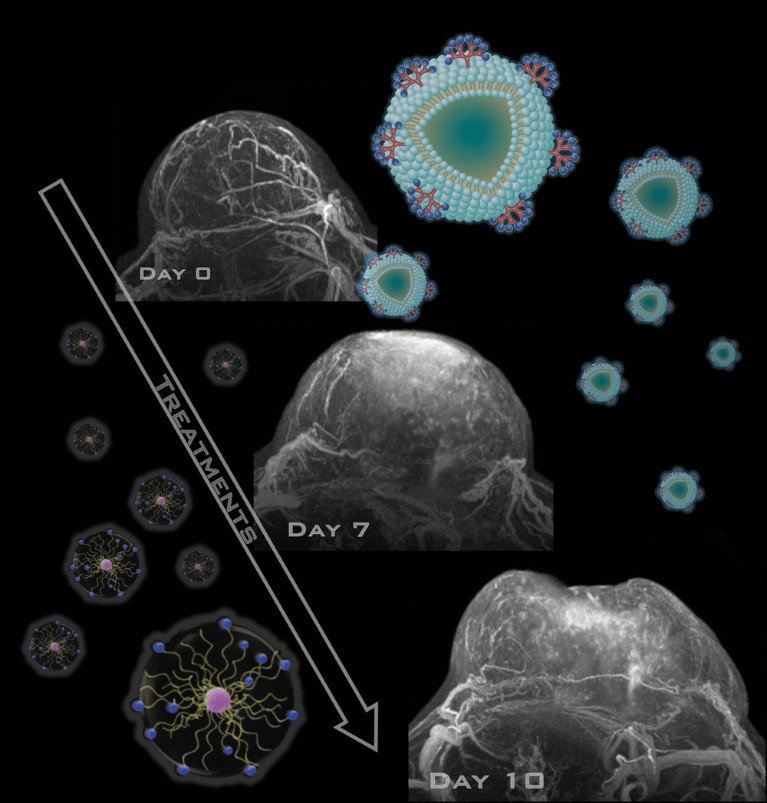
In solid tumours, the contrast was 131% above that of normal tissue after 30 minutes, much higher than seen with another clinically approved contrast agent, Gd-DTPA. After an hour, the tumour-to-normal ratio was 150% and enhanced to over 170% at the low pH areas for several hours. This indicates to researchers that the released Mn2+ of the nanoparticle is attaching to the cancer cell via protein binding, helping to provide better contrast.
“This sort of in vivo nanoparticle imaging is important,” says Aoki. “It allows confirmation of delivery concentration before treatment, as well as selection of the optimum treatment method. It helps us select the size and property of nano-platforms that can be delivered, and to predict their therapeutic efficacy. And if accumulation at the delivery site is inadequate, we can consider combination therapy, such as particle beam radiotherapy or photodynamic therapy.”
But the approaches are not limited to cancer, he says. “Aneurysms, arteriosclerosis and myocardial ischemia can be targeted using nanoparticle-based drug delivery systems with MRI. Since research on nanoparticle delivery into the brain is advancing, neural disorders — including dementia and cerebral infarction — can be targeted.
“We’re also working on new medical therapies that allow treatment while monitoring, by combining MRI and nanoparticle technologies,” says Aoki.
Theranostics — the combination of targeted therapy based on targeted diagnostic tests — promises to make the dream of personalized medicine a reality. QST, by verifying the efficacy of new approaches in preclinical research and developing diagnostic technologies with higher precision, is helping create that future using nanoparticle-enhanced MRI, PET/SPECT, optical imaging, laser technologies, charged particle therapy and isotope therapy. These nanoscale diagnostic and therapeutic tools are allowing clinicians to better understand a patient’s disease and design optimized, individual treatments.
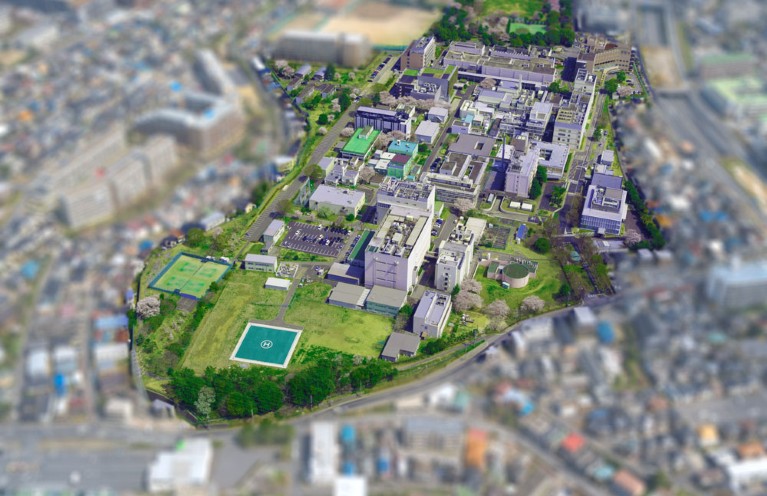
Headquarters of QST at the National Institute of Radiological Sciences, located in the Tokyo-bay area between the two international airports, Narita and Haneda.
Moreover, QST’s work is helping accelerate the novel field of ‘quantum life science’, which aims to advance our understanding of physiological and biological processes with research tools based on quantum technologies. One team is seeking to improve the sensitivity of MRI technology by combining hyperpolarization with nitrogen-vacancy centres. Hyperpolarization is a technology that increases signal enhancement by 100,000 at the cellular level. Nitrogen-vacancy centres are point defects in diamonds that make possible photoluminescence and hyperpolarization at room temperature.
For all of these, advances will be needed in image analysis and measurement, some of it relying on artificial intelligence, also under development at Aoki’s lab. Ultimately, his hope is for imaging to be non-invasive, low-cost and easy to access. This would allow us to diagnose diseases with a high level of precision before they become serious and then provide early intervention with imaging and nanomedicine.



 Focal Point on Nanomedicine in Japan
Focal Point on Nanomedicine in Japan