The blood-brain barrier is nature’s border police: preventing microbes and damaging substances from entering the central nervous system. But it also thwarts medicines that could treat tumours, Alzheimer’s and other diseases affecting the brain.
While there’s a global effort underway to treat ailments in the brain by developing biomolecules such as antibodies, peptides and nucleic acid agents, these are mostly too large to cross the blood-brain barrier, says Dr Mariko Tosu, a veteran pharmaceutical executive and former researcher who was appointed Braizon’s CEO in March 2017.
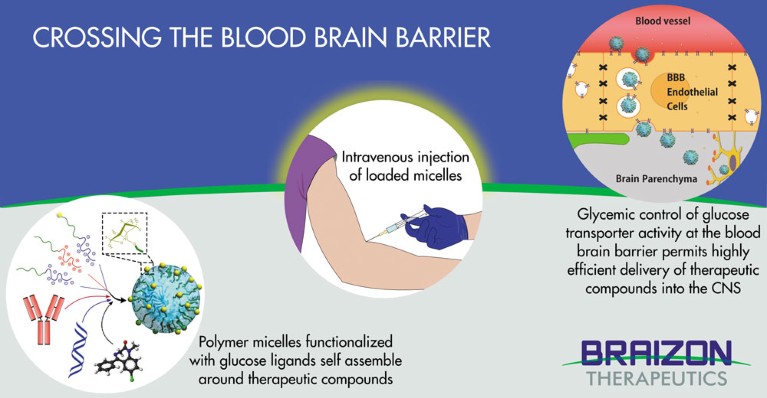
Current efforts are focused on infiltrating the blood-brain barrier by hijacking its active transport receptors — such as those used by insulin or transferrin — to carry drugs into the brain. But results in animal trials have often been disappointing, with either too little active compound reaching neurons or not enough persisting to be useful. Now, Japan’s Braizon Therapeutics Inc has found a way through the blood-brain barrier by piggybacking on the one molecular pathway that the brain most hungers for: glucose.
“We discovered that we can manipulate the transporter of glucose, one of the most highly expressed proteins at the blood-brain barrier,” says Dr Philip Davy, a senior scientist at Braizon who now manages the company’s business development. “And by manipulating glycaemic control, we can boost the amount of substrate the nanotechnology packaging system delivers through the blood-brain barrier by up to 100 times.”

Novel polymer micelle carrier research
Braizon’s technology is the first to not only successfully manipulate the glucose transporter GLUT1 — the brain’s primary energy pathway — but also to deliver substantial quantities of useful molecules to the neurons of animal models.
Braizon relies on polymer ‘nanocarriers’, or micelles, developed at the University of Tokyo and the Innovation Center of NanoMedicine. With a diameter of 20 to 50 nanometres, the micelles have their surfaces adorned with precisely controlled glucose densities. Each micelle can carry a payload of a range of active pharmaceutical ingredients, from low molecular weight drugs to biomolecules such as nucleic acid drugs, antibodies and proteins.
Although glucose transporters are also used in other organs, such as muscles or the spleen, the boost in the brain obtained by using polymer micelles and glycaemic control is dramatic. That’s partly because, after fasting, the brain demands a rapid uptake of glucose for energy; as well as the fact that it is a very greedy organ, taking 20 to 25% of the body’s glucose at any one time.
“Usually, most GLUT1 locates to the abluminal site, or inside the brain capillary endothelial cells (BCEC) in blood vessels,” explains Tosu. “But it migrates to the luminal site of BCEC when fasting to transport glucose effectively into the brain following the increased supply of glucose that comes from eating a meal. Braizon’s technology utilizes this mechanism to facilitate the transport of glucose-decorated micelles into the brain.
“This is a brain-specific drug delivery system,” says Tosu. “The combination of this mechanism with tunable nano-micelles has the versatility to include a wide range of small molecules and biopharmaceuticals for delivery into the central nervous system, something never previously achieved with a commercial delivery platform.”
In addition to cloaking the micelle in glucose, the technology takes advantage of PEGylation, the process of attaching strands of polyethylene glycol (PEG) polymer chains to the active drug molecules. The covalent attachment of PEG to a drug or therapeutic protein can help ‘mask’ it from the immune system, boosting its size in solution.
“When you coat something with PEG and put it in the bloodstream, it protects the micelle from being tagged by the immune system for removal,” says Davy. “So you increase the persistence of the compound in circulation, and reduce the rate at which it’s removed, which are actually two separate benefits.
“The other advantage is when the drug crosses over the barrier, it’s still encapsulated in the micelle, and doesn’t break down until it gets to where it’s supposed to be. So, not only is it getting into the brain, but it’s actually moving toward targeted cells in brain parenchyma and arriving intact, before the therapeutic payload has been released,” he adds. “Using our system, you can get up to 6% of a systemically delivered dose into the brain, which is huge.”
Regulatory approval for human use is still ahead, but patient trials of polymer micelle transportation systems — first developed for use in cancer therapy — have already been completed. And PEG polymers have been used in a large variety of human-rated products for decades.

Team Braizon discusses delivery strategies for a new contract
Braizon, a US$6.7 million private company whose backers include Japanese venture capitalists Fast Track Initiative, Inc and SMBC Venture Capital Co Ltd as well as UTokyo Innovation Platform Co Ltd, is opening research facilities in Boston to add to its R&D members working at the Innovation Center of NanoMedicine in Kawasaki, and plans to go public in 2024.



 Focal Point on Nanomedicine in Japan
Focal Point on Nanomedicine in Japan