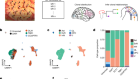
Scientists at the African Centre of Excellence for Genomics of Infectious Diseases in Ede, Nigeria, prepare samples for DNA sequencing.Credit: ACEGID
In 2020, an analysis of 426 African genomes, involving researchers from 15 African countries, uncovered 3 million new variants in the human genome1. The discovery contributed to the development of a tool that enables researchers to identify genetic associations specifically in African populations — the Infinium H3Africa Consortium genotyping array, produced by the US biotechnology firm Illumina.
Although various enterprises have supported cutting-edge human genomics in Africa, the Human Heredity and Health in Africa (H3Africa) initiative2, which supported this work, has probably contributed the most in terms of infrastructure and training. The US$176-million programme began in 2010, funded by the US National Institutes of Health (NIH) and the UK biomedical charity Wellcome (in partnership with the African Society of Human Genetics). Projects have ranged from population-based genomic studies of common disorders, such as heart disease, to investigations of infectious diseases, such as COVID-19. Together, some 51 projects, all led by African scientists and involving researchers from more than 30 African countries, have resulted in 50,000 samples being genotyped and nearly 700 papers being published.
Thanks to H3Africa and other genomics initiatives, such as the Nigerian 100K Genome Project3, African genomics is now poised to improve the health of millions of people worldwide, including those across the continent and the African diaspora. But building on the discoveries made so far — and especially applying findings to the clinic — will require several systemic changes, including a major shift in how genomics research in Africa is funded.
All remaining projects supported by the H3Africa initiative are expected to wrap up this year. (Although funding formally ended in June 2022, some H3Africa grant recipients were able to obtain extensions because of disruption from the COVID-19 pandemic.) Here, we lay out what is needed to ensure that investment in genomics in Africa is not just sustained in a post-H3Africa world but expanded. In our view, Africa could become the birthplace for a new kind of genomics — one that brings better health to all.
What H3Africa has achieved
As of 2021, nearly 86% of participants in genome-wide association studies (GWAS) worldwide were of European descent, even though that group makes up only 16% of the world’s population4. (Such studies screen the genomes of thousands of people to establish whether a particular genetic variant is associated with a trait of interest.) This bias means that precision-medicine tools, such as polygenic risk scores — which use genetic data to predict a person’s risk of developing a certain disease — are much more accurate for people of European ancestry5.
H3Africa has helped to address this inequity in at least five ways.
First, H3Africa has made it easier for Africans to pursue genomics on the continent (see ‘Tapping the potential’). This has been made possible through establishing sequencing facilities, including in South Africa, Gambia, Nigeria, Ethiopia, Uganda and Botswana, and setting up training programmes, for example in bioinformatics6. Because African institutions were made the direct recipients of grants in the H3Africa programme, Africans have been able to design projects, training and infrastructure according to the needs of their own countries.

Source: PubMed/Analysis by Z. Lombard
Second, H3Africa has increased the profile of African genomics globally. In part, this is thanks to researchers and organizations funded by H3Africa partnering with international bodies, such as the Heads of International Research Organizations (HIRO — composed of the directors of those organizations, including major funders such as the NIH and the Bill & Melinda Gates Foundation).
Third, H3Africa has enhanced education and awareness about genomics across the continent — principally by requiring that all projects involving participants include some amount of community engagement. For example, Genome Adventures, an initiative of an H3Africa-funded network of organizations called the Collaborative African Genomics Network, is likely to have contributed to the high retention rate of participants in the network’s research project. In this case, workshops, comic books and social media were used to educate community stakeholders and the public about genomics and biomedical research.
Fourth, H3Africa has enabled an unprecedented degree of scientific collaboration between researchers — both across the continent and with those elsewhere. It achieved this partly by requiring that funded African researchers collaborate with other African scientists. During the COVID-19 pandemic, for example, researchers at the African Centre of Excellence for Genomics of Infectious Diseases (ACEGID) in Ede, Nigeria, trained more than 1,300 geneticists, public-health workers and officials from other African countries in diagnostics, next-generation sequencing and bioinformatics. (ACEGID, established in 2014, has received around 30% of its funding from H3Africa.)
South Africa’s San people issue ethics code to scientists
Lastly, by providing ethics guidelines that are tailored to African settings, H3Africa has helped to establish standards and norms around data sharing7 and the communication of findings to study participants. Although the rules and regulations on this vary greatly across Africa’s 54 countries, geneticists across the continent now have a central resource for guidance on informed consent, stigma around genetic diseases, the sharing of benefits from studies and other topics.
Realistic goals
Although most H3Africa projects have not yet translated findings into clinical practice, some have already had real-world impact. For instance, over the past eight years, work at ACEGID has informed public-health responses to Lassa fever, Ebola and COVID-19. Likewise, to improve paediatric care, Solomon Ofori-Acquah, an H3Africa-funded researcher at the University of Pittsburgh in Pennsylvania and at the University of Ghana in Accra, last year established a genetic-counselling training programme in Ghana and paired it with an existing genetic screening programme for newborns.
But will all this progress in African genomics be sustained after H3Africa funding ends? What is needed to ensure that current and future investments translate into improved health for people?

The Collaborative African Genomics Network, part of the H3Africa consortium, produced a set of four Genome Adventures comic books to improve public understanding of genomics in Botswana.Credit: CAfGEN Genome Adventures/Botswana-Baylor
In some areas, it is easy to imagine how real-world genomics applications in Africa could be ramped up. Building a continent-wide early-warning system to detect and track disease outbreaks, for instance, could be achieved largely through scaling up the ACEGID model. An array of such centres across the continent would enable training of African scientists, help to retain African talent in Africa and ensure that standards of practice around African genomic surveillance are developed on the continent.
The Africa Centres for Disease Control and Prevention has already established a surveillance network called the Pathogen Genomics Initiative. This consists of national public health institutes and genomics laboratories, such as ACEGID.
In other areas, the health benefits are farther away. Examples include treatment of common, non-infectious conditions (such as diabetes), largely because of a lack of data, and the treatment of rare diseases, largely because of a lack of genomic medicine services. However, it is becoming clearer what needs to happen next.
Because African genomes have a longer evolutionary history and harbour more variation than the genomes of people with European ancestry, they offer a richer source of variants linked to traits of interest, such as rare developmental disorders. Studies are revealing the incredible potential of African genomes: for developing ways to diagnose diseases across diverse populations; uncovering new therapeutic targets; and identifying genetic markers that indicate how someone might respond to a particular drug.
As an example, throughout the world, a mutation in a gene on chromosome 4 is used to diagnose Huntington’s disease. However, another form of the disease exists that is clinically indistinguishable, called Huntington’s disease-like 2 (HDL2). This is caused by a mutation in a gene on chromosome 168,9. So far, all cases of HDL2 have been found in people with African ancestry. This suggests that individuals who have Huntington’s disease symptoms but do not have the chromosome 4 mutation should be tested for the mutation on chromosome 16.
Read the paper: High-depth African genomes inform human migration and health
To deliver the greatest yield per investment when it comes to looking for translatable findings in African genomes, a network of genomic centres needs to be established across Africa. Geneticists operating in such a network should collaborate with those working on multi-year cohort studies to tease apart the effects of genetic and environmental factors, particularly for complex diseases.
One encouraging sign is a funding award to help establish the African Population Cohort Consortium. Last year, the African Population and Health Research Center in Nairobi received funding from Wellcome to develop and co-lead this consortium with the Africa Health Research Institute in Durban, South Africa. It will collate health-surveillance data and biospecimens to provide a resource for large-scale population studies.
Ideally, investment in an African genome initiative at scale would happen alongside the establishment of a pan-African biobank similar to the UK Biobank, which has been periodically collecting health and genetic information from more than 500,000 participants since 2006. H3Africa has already established biorepositories in Uganda, Nigeria and South Africa, which involve the collection of samples linked to the H3Africa genetic data resource. The Genetics of Latin American Diversity (GLAD) Project10, which includes genome-wide data on around 54,000 individuals from 39 studies, shows that such a standardized, pan-continental approach is feasible.
Systemic changes needed
In July last year, the World Health Organization (WHO) Science Council — which advises the WHO director-general on high-priority scientific issues — stressed the importance of genomics to future global health in a special report11. In our view, heeding the WHO’s call and realizing the goals laid out here will require four systemic changes to genomics research in Africa.
Government investment. African governments have been slow to support genomics infrastructure. None of them has delivered on pledges made at the 2007 African Union assembly to spend 1% of gross domestic product on research and development by 2010 (see ref. 12 and go.nature.com/3jt9dss).

Participants in H3Africa’s Deciphering Developmental Disorders in Africa research project attend a community Information session in the Democratic Republic of the Congo.Credit: Prince Makay
Convincing political leaders in Africa to invest in genomics has been challenging, in part because it can take decades before pay-offs are realized. The fact that most evidence for the benefits of genomics comes from research on European-ancestry populations in high-income settings also makes it harder for scientists to convince African governments that genomics could help their own people.
Yet as long as researchers rely on grants from outside funders such as the NIH, African governments won’t have ownership of projects or be able to set priorities. What’s more, sustained government commitment to genomics, which would enable researchers and health-care practitioners to keep working in Africa, is crucial to bringing the benefits of genomic medicine to local populations.
Matched funding schemes, whereby funds are provided by a donor on the condition that the receiver also contributes resources (similar to those implemented by the UK Newton Fund in selected African, Asian and South American countries), could help to shift trends in Africa away from over-reliance on donorship.
Industry buy-in. South Africa houses around one-quarter of all the next-generation sequencing facilities on the continent13. For genomic medicine to have an impact on the health of millions, data need to be generated that are of similar quality to those used in the global north, and they must be produced at a similar pace and price.
Two years of COVID-19 in Africa: lessons for the world
More government investment would help with this. Public–private partnerships are also key. Major pharmaceutical companies are increasingly expressing interest in scaling up translational genomics in Africa. At the American Society of Human Genetics annual meeting last October, for example, an emerging consortium of seven firms met with H3Africa investigators to discuss how current fragmented initiatives might be transformed into an integrated public–private partnership.
The responsible engagement of industry — based on principles of African ownership, and the equitable distribution of credit and benefits — could help to resolve crucial needs, such as the lack of maintenance staff for sequencing machines, affordable reagents and reliable supply chains.
Various endeavours have already demonstrated the promise of public–private partnerships. For instance, a genomics centre was established in 2018 in Cape Town, South Africa, thanks to a partnership between the Chinese genomics company BGI Group and the South African Medical Research Council. It was one of the facilities used to detect Omicron SARS-CoV-2 variants in South Africa’s wastewater using high-throughput sequencing. Similarly, in 2021, the Illumina and Genetic Alliance, a non-profit organization that advocates research on rare diseases, launched the iHope Genetic Health programme. This $120-million initiative aims to expand global access to whole-genome sequencing, with more than one-third of the money going to Africa. But such alliances need to be much more widespread.
Genetic services and electronic health records. In the United States, there are 2 medical geneticists and 7 genetic counsellors for every 500,000 people14. In South Africa, which offers the most extensive medical genetics services in Africa, both these numbers are less than 0.2 (see go.nature.com/3yjoxpk and ref. 15).
For people in Africa to benefit from potentially transformative genomic medicine applications, such as emerging gene-based therapies for sickle-cell disease, genetic counselling services are needed in every country. (Even with limited screening, sickle-cell disease is known to affect around 225,000 babies in Africa annually16. By our estimate, the disease results in 8–15 deaths per 1,000 children under 5 years old.) Electronic health-record systems are also essential if the use of genomic information in clinical care is to become routine.

A physician in Cameroon discusses prenatal testing with a patient. Such screening for genetic diseases is limited across Africa.Credit: Heiner Heine/imageBROKER/Shutterstock
Equitable science and meaningful engagement of research participants. The legacies of colonialism and scientific racism continue to block opportunities for African scientists to participate equitably in genomics17. Research that focuses on the diseases most prevalent in Africa are often led by non-African organizations, for instance. Researchers globally have frequently failed to consider how benefits stemming from their work could be shared equitably, and some genome data have been used unethically18,19.
Changing this will require a commitment from all stakeholders to promote equitable collaboration with partners in Africa, modelled on the successes of programmes such as H3Africa. A broader array of funders, including major philanthropic organizations, needs to support genomics initiatives on the continent. Priority should be given to proposals for projects that are led by African scientists, that represent African populations and that focus on research questions pertinent to African communities.
To ensure that research is shaped according to the priorities of people living in Africa, engagement with research participants must also be tailored to specific cultures and languages. Some 2,000 languages are spoken in Africa, representing a significant barrier to communication. The Lyfe Languages initiative, a project developed in Western Australia to help overcome a barrier between Aboriginal people and health-care providers, offers one model for how this might be achieved. The project provides Indigenous-language translations of terms often used in clinical-genetics research20.
The next decade
Some researchers who have been reliant on H3Africa funds are in a strong position to compete for the global pot of money available for genomics research. This includes that offered by the NIH and the US National Science Foundation. Many have been awarded grants to join a new consortium called Data Science for Health Discovery and Innovation in Africa (DS-I Africa). This effort, which is focused on data science and analysis, builds on H3ABioNet, H3Africa’s bioinformatics training programme, and is being funded for up to ten years by the NIH Common Fund Initiative.
Most researchers urgently need African governments and other funders to step up to help them secure a viable future in research on the continent. What’s more, government commitment is crucial to securing a sustainable future for personalized medicine and medical genetics services in African countries.
This is an opportune moment for a ‘moonshot’ enterprise to advance the potential of genomics in Africa. A group of H3Africa investigators and NIH colleagues have come together to propose a programme of Genomics Centres of Excellence. These could absorb multiple genomics projects, scale up training, adopt common standards and, through institutional strengthening, offer greater sustainability than sponsorship of individual projects alone. The plan will be presented later this month at the International Congress of Human Genetics in Cape Town, South Africa.
An incredible amount has been achieved in little more than ten years. But with the right investment from the right stakeholders, Africa could achieve so much more in genomics in the decade ahead — with benefits that reach far beyond the continent.

 Two years of COVID-19 in Africa: lessons for the world
Two years of COVID-19 in Africa: lessons for the world
 South Africa’s San people issue ethics code to scientists
South Africa’s San people issue ethics code to scientists
 Read the paper: High-depth African genomes inform human migration and health
Read the paper: High-depth African genomes inform human migration and health