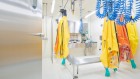
Grace Nambatya Kyeyune oversees research on natural products at the Natural Chemotherapeutics Research Institute in Kampala.Credit: Tumuhimbise Harrison
Grace Nambatya Kyeyune is a natural-products research scientist and director of research at the Natural Chemotherapeutics Research Institute (NCRI) in Kampala, which is a part of the Ugandan Ministry of Health that is dedicated to evaluating traditional medicines. She graduated with a bachelor’s degree in chemistry from Makerere University in Kampala in 1984, and then joined the NCRI as a scientific officer in the chemistry division. But it was a bout of eczema, and a failed herbal treatment for it, that motivated her to dive deeper into medicinal chemistry, earning a master’s in 1989 and then a PhD in 1993 at Loughborough University, UK. There, she learnt methods for extracting drugs from herbs and identifying their mode of action in humans. Now, she is one of the leading natural-products researchers in Uganda, overseeing the evaluation of medicinal plants for treatment efficacy and safety at the NCRI.
How did you first get interested in traditional medicine and using modern scientific methods to study them?
My grandfather, like other Africans of his time, used traditional medicine to treat several ailments. As children, we would be given baths in an extract made from the leaves of the omwoloola tree (Entada abyssinica) to treat skin infections, among other conditions.
But my professional interest in traditional medicine was inspired by an eczema rash on my feet that left patches of black and white on my body when I was a university student. I hated the patches on my body. The use of modern steroid creams such as hydrocortisone failed to heal the rash. As a chemist who also had traditional knowledge, I was motivated to try traditional herbs. I visited a popular herbal clinic at the time in Kampala, with the hope that my skin challenge would be addressed. However, the results were devastating; the side effects of the herbal remedy were worse than the rash. It was bad news. This experience, and the lack of regulation of these products, motivated me to be part of those working to improve the development of traditional medicine.
How is the NCRI helping to ensure that these products are safe and effective?
All I can say is that we’re progressing. We have a law, called the Traditional and Complementary Medicine bill, that sets out the procedures for the development and promotion of traditional medicine. We have trained some herbalists in best practices of conservation of natural resources, hygiene and quality assurance in the herbal processing chain. We have a laboratory at the NCRI where we validate the products for safety, in part by conducting a phytochemical screen to ascertain the key ingredients.
We see positive results. Uganda’s National Drug Authority has registered more than 230 natural products that are on the market in the authority’s database.
Career resources for African scientists
We have also partnered with Mulago National Referral Hospital in Kampala, the largest public hospital in the country, to test some of our traditional-medicine products in phase I and II clinical trials through the Clinical Trial Centre for Natural Products there.
For example, together with a diverse team of scientists, we are evaluating whether a compound called UBV-01N, which is used to manage viral infections, could be used in the management of COVID-19 symptoms.
You did a lot of benchmarking in several countries. What did you learn that you want to incorporate into traditional medicine in Uganda?
We visited Ghana and South Africa on the continent, and China, South Korea and India in Asia. These countries have a long tradition of using traditional medicine. It is integrated into their food cultures and daily living. There is a bachelor’s degree in herbal medicine at the Kwame Nkrumah University of Science and Technology in Kumasi, Ghana. In South Africa, we forged a partnership that also gives us access to their laboratory technologies, such as tissue culture for our product development. From South Korea, we have learnt ways of propagating herbs in the laboratory for standardized biomass production for natural pharmaceuticals.
With funding for clinical trials lacking in many African countries, do you think it is more important to test new cancer drugs or other pharmaceuticals, or to run trials of traditional medicines that are commonly used and more affordable?
I think it is better to run trials on traditional medicines that are already in use by our communities and have been used for generations with a great deal of success. Many of the traditional medicines are scientifically sound treatments. Even the large pharmaceutical companies are picking them from here to be developed into drugs, such as the Ugandan greenheart tree (Warbugia ugandensis), extracts of which have been used to treat coughs and malaria.
What we are promoting is standardization; to develop the right dose and delivery method for safety and effectiveness. This will also support the development of our economy, because we would get World Health Organization certification for export.
How will intellectual-property rights be dealt with considering that many of these medicines are based on traditional knowledge passed on from generation to generation?
We work with the Uganda Registration Services Bureau. It has created a traditional-knowledge database. This is the first point of reference for anyone interested in the development and promotion of traditional medicine in Uganda. There is information on existing patents on traditional medicines, traditional medicine benefit-sharing, documentation of traditional medicine, and which communities own the rights to develop or share benefits in the development of particular traditional medicines.

 My life at the helm of a top African cancer-treatment centre
My life at the helm of a top African cancer-treatment centre
 Harmony on the farm: integrating crops, trees, goats and bees
Harmony on the farm: integrating crops, trees, goats and bees