Credit: Sally Evans
David (Dave) Evans discovered reactions that he applied to the synthesis of biologically active natural products as potential drug therapies, including antibiotics and anti-cancer agents. His contributions changed how researchers make complex natural molecules and design drugs. He has died, aged 81.
Evans was a devoted educator who invested enormous effort in scientific communication. In the mid-1980s, he recognized that students and researchers faced challenges in producing graphics for oral presentations, manuscripts, theses and grants using drafting pens and templates. Evans made the connection between the graphics capabilities of the Macintosh computer and the graphics-heavy nature of organic chemistry. Along with his wife Sally and graduate student Stewart Rubenstein, he developed ChemDraw. Organic and inorganic chemists around the world, in academia and industry, now consider this chemical-structure-drawing software essential. By transforming how organic chemistry is presented and taught, Evans influenced successive generations far beyond the walls of his institutions.
Evans was born in Washington DC. He earned a scholarship to Oberlin College, a small liberal-arts university in Ohio. There, the chemist Norman Craig engaged him in his research on small organic molecules. Evans forged a life-long friendship with Craig and remained dedicated to Oberlin. He often shared the story that he had cut the grass at the Craigs’ home one Saturday so that Craig could do some glass-blowing to make equipment for an experiment of mutual interest.
Those early studies shaped Evans’s approach to problems in organic chemistry throughout his career. He combined an intuition for chemistry with a determination to understand the origins and selectivity of reactions.
Evans began his doctoral studies at the University of Michigan in Ann Arbor and completed his PhD at the California Institute of Technology in Pasadena. He accepted his first faculty position in 1967 at the University of California, Los Angeles, later becoming a full professor. There, he began to study rearrangement reactions, in which the number and type of atoms remain the same, but their connectivity changes. Rearrangement processes are desirable in organic synthesis because many of them proceed through well-organized transition states that make it possible to plan the synthesis of complex molecules. Evans and Kurt Mislow at Princeton University in New Jersey both reported studies on an atomic-bond rearrangement that became useful in the synthesis of hormones and natural toxins.
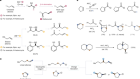
Evans also discovered a remarkable way to accelerate a rearrangement reaction that simultaneously makes and breaks carbon–carbon bonds while controlling stereochemistry, known as the Evans anionic oxy-Cope reaction. Arthur Cope first reported on this reaction in the 1940s, but it required temperatures in excess of 200 °C. Evans identified starting materials that allowed the reaction to occur under much milder conditions, widely applicable in organic synthesis. One of several named reactions associated with Evans, it became the subject of intense investigation in an effort to understand the origin of its acceleration rate.
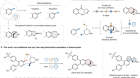
Evans returned to Caltech in 1974 and remained there until 1983. During this period, he made what are viewed as his most impactful discoveries, which fuelled decades of further work. The aldol reaction had been known since 1869, but a practical way to control the relative and absolute stereochemistry was missing. Evans demonstrated that the use of boron created highly organized transition states that overcame these limitations. He invented an ‘auxiliary’ as a chiral agent, prepared from a readily available amino acid. Interaction of the boron enolate with an aldehyde creates motifs found in many natural products with biological activity. Academia and industry embraced this methodology to make natural products and therapeutics of commercial interest.
Evans’s last academic stop was at Harvard University in Cambridge, Massachusetts, where he was briefly chair of the chemistry department. His group revealed the full synthetic potential of the Evans auxiliary during this period, preparing numerous complex natural products. They also made major contributions to asymmetric catalysis. These syntheses tackled thorny assemblies of stereochemically complex components. Among the most significant contributions were total syntheses of the antibiotic vancomycin and the anti-cancer agents bryostatin 2 and spongistatin 2.
Evans put the same level of effort into his lecture notes as into his scientific conference presentations. He made those notes available to the chemistry-education community, which adopted and modified them, influencing the teaching of subsequent generations. For years, he kept a database of reaction mechanisms and synthesis problems on a publicly available website; this came to be used worldwide. He trained many scholars, including postdoc David MacMillan, who received a share of the 2021 Nobel Prize in Chemistry for his work on asymmetric catalysis of organic molecules. Evans’s first female doctoral graduate, Ann Weber, became a vice-president of lead optimization at the US-based pharmaceutical company Merck and is recognized for her role in the discovery of the diabetes medication Januvia (sitagliptin).
Dave Evans had an uncanny appreciation of chemistry’s overarching synthetic challenges, and he was supportive in framing a problem in such a way that the creativity of each individual was best expressed and harnessed. He leaves behind some of the finest synthetic sequences ever devised for making nature’s most complex naturally occurring bioactive molecules.


 Robert Grubbs (1942–2021)
Robert Grubbs (1942–2021)