THE PAPER IN BRIEF
• Some genes are constrained, which means that damaging variants of them are removed from the population by natural selection.
• Writing in Nature, Gardner et al.1 investigated the processes underlying this evolutionary process in humans.
• They report that having a high overall amount of damaging genetic variation in constrained genes is associated with childlessness in men.
• The association is linked to only 1% of the chance of childlessness between individuals, but to larger effects over many generations in a population.
• The findings are consistent with the hypothesis that having a greater burden of damaging genetic variation might affect a man’s ability to find a mating partner.
LOIC YENGO: An evolving understanding of gene constraint
Loss-of-function (LoF) mutations inactivate genes completely. Some genes in a human population are able to ‘tolerate’ LoF mutations, whereas others, known as constrained genes, cannot — LoF variants in constrained genes tend to be lost over time through natural selection. As a result, fewer people would be expected to have LoF variants in a constrained gene than in an LoF-tolerant gene. A large study of human genetic variation carried out by Lek et al. in 2016 identified about 3,000 LoF-intolerant genes2. Gardner and colleagues’ work might help us to understand how natural selection has constrained them.
Read the paper: Reduced reproductive success is associated with selective constraint on human genes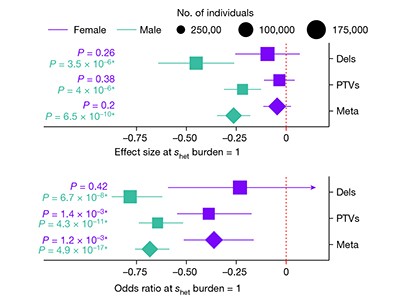
It is thought that LoF mutations might affect reproductive fitness — that is, the number of offspring an individual produces. For example, these mutations might reduce the chance of a person living to reproductive age, cause infertility or affect a person’s ability to find a mate. About one-third of the 3,000 constrained genes identified in Lek and colleagues’ study have been linked to disorders associated with mortality before the individual reaches reproductive age and with reduced fertility (according to the Online Mendelian Inheritance in Man database; https://omim.org). But whether and how the other genes might affect reproductive fitness has been unclear.
To address this issue, Gardner and colleagues analysed rare protein-truncating mutations in the 3,000 genes in more than 300,000 unrelated individuals who are part of the UK Biobank — a database of genetic and health-related information for 500,000 volunteers in the United Kingdom. This cohort is made up largely of individuals between 39 and 73 years old, ensuring that most have, in principle, had the opportunity to reproduce. The authors quantified the overall association of all protein-truncating mutations and gene deletions with reproductive success. Their main finding is that, cumulatively, LoF variants in these 3,000 genes are associated with childlessness in men but not in women. Interestingly, this association is not mainly driven by reproductive dysfunction. Instead, men with a higher number of deleterious mutations in these genes are more likely than those with fewer numbers to exhibit behavioural and cognitive traits that might reduce their likelihood of finding a partner.
One of the key implications of these findings is that cultural practices such as mate choice might have had a prominent role in the course of natural selection. The association between mutations constrained in our distant past and modern human behaviours could be a fundamental discovery, suggesting that traits associated with mate choice are the same today as they were thousands of generations ago.
But for studies such as those of Gardner et al. and Lek et al., the sample size determines the evolutionary period that the study can tell us about. For example, larger samples are needed to track more-recent natural selection, because more-recent genetic changes will be present in fewer individuals3. And there is a near-sixfold difference in sample size between these two studies. This suggests that the evolutionary period over which the 3,000 genes became constrained might differ from the time over which natural selection has produced the genetic associations with reproductive fitness observed in the UK Biobank. This is a caveat that can weaken Gardner and colleagues’ conclusions. But it could be addressed in the future by using a larger, updated list of constrained genes4.
Overall, Gardner and colleagues’ findings seem to be consistent with Charles Darwin’s still-debated theory of sexual selection5, which posits that reproductive fitness is partly driven by within-sex competition and mate-choice preferences between sexes. It is important to note, however, that the current associations involve only a limited number of (genetic) factors, which are not sufficient to explain all causes of childlessness. More evidence for the mechanisms underpinning Gardner and colleagues’ findings is needed, through replication in other populations (and species), in which mate choice could be influenced by different cultural standards from those of ancestors of the contemporary UK population. This should improve our understanding of the mutational constraints that shape our evolution.
HEIDI COLLERAN: Mate choice beyond genetics
About 20% of people in the United Kingdom never have biological children6. Many interacting social, biological and demographic factors contribute to the dynamics of childlessness, whether or not a person is childless by choice. Gardner and colleagues’ work highlights one element of this complexity, pointing to a possible connection between human behaviour and genetics as a component of childlessness.
Sex chromosomes manipulate mate choice
The authors performed a battery of genetic-association analyses that indicate that men (but not women) who have a high number of rare damaging genetic variants are slightly less likely to have children than are those with fewer of these variants. A range of other associations exist, too, although the effects are small in all cases. For instance, both men and women with the highest number of variants are more likely to score lower in intelligence tests than are people with a lower number. They are less likely to have university degrees or high incomes, and are more likely to be socio-economically deprived or have mental-health conditions. The strongest correlation is related to being single: men with a high number of damaging variants were more likely than comparable women to be living alone at the time of data collection.
These results explain less than 1% of the variation in reproductive outcomes between individuals. But, compounded over many generations, the authors infer, this sex difference means that about 20% of the selective pressures that act on these genes as they evolve are attributable to sexual selection — that is, to female mate choice.
First, some crucial caveats. Causality cannot be inferred from any of Gardner and colleagues’ results, given the associative nature of the analyses. And even men with the highest numbers of genetic variants have a 50% chance of reproducing, so these aren’t ‘genes for childlessness’. In addition, the authors have only a snapshot of each individual’s relationship status at the time of recruitment to the UK Biobank, and no information about the type or length of their current or past relationships, or their partnership and reproductive preferences. Taken together, these factors would paint a more complex picture of each individual’s life, making it harder to draw strong conclusions from these correlations.
Some demographic evidence corroborates Gardner and colleagues’ findings: across 13 European countries, men with low incomes are more likely to be childless7. But broader demographic factors, which don’t always affect men and women symmetrically, also play a part. There are typically more male children born than female. Men more often father children with multiple women than the reverse, and are regularly older than their reproductive partners. In declining populations, these differences can leave a larger proportion of men without partners8.
Thousands of human sequences provide deep insight into single genomes
Unpicking many interrelated factors to tease out why men might forgo or miss out on having children is a huge challenge. For instance, poverty has adverse effects on both academic achievement and mental health9,10. Broader social and cultural influences on partnership and reproductive preferences will surely outweigh the 1% of variance explainable by genetic differences.
In a simplified world, in which sexual selection alone determines the chances of having children, one possible assumption might be that men desire sex and reproduction, and that women choose mates who are wealthy and (in contemporary economies) well educated. Gardner and colleagues’ work is centred around the genetic outcomes of such a potential scenario, but it is risky to interpret the findings too strongly. We must be careful not to reduce childlessness to a deviation from a presumed (hetero-)norm. To do so plays into problematic narratives about the ‘childless’ poor being lonely and unwanted, or the ‘child-free’ rich being selfish.
In fact, there are many more dimensions to mate choice. First, priorities vary between individuals and over time. Although some women might seek educated, neurotypical men to father their (increasingly few) desired children, evidence suggests that, in the United Kingdom, more women currently prioritize kindness and supportiveness (see go.nature.com/3hnvxlv). Second, individuals don’t always freely choose their reproductive partners. Arranged marriages, still common today, were historically widespread. Darwin’s own marriage didn’t clearly follow straightforward sexual-selection criteria: his cousin (and sister-in-law) Emma Wedgwood accepted his proposal largely because of a mutual goal of family consolidation11. The cross-cultural variation in human mating and marriage practices is vast — inferring human universals from just one population would therefore be premature.
Although the genetics here have little predictive power on an individual level, a key message is that the ways in which we socially construct and organize our biological reproduction — which are at the heart of human social life — might have important genetic legacies at the population level and over many generations. A better understanding of the co-evolution of reproduction, culture and genetics is now crucial12.

 Read the paper: Reduced reproductive success is associated with selective constraint on human genes
Read the paper: Reduced reproductive success is associated with selective constraint on human genes
 Thousands of human sequences provide deep insight into single genomes
Thousands of human sequences provide deep insight into single genomes
 Sex chromosomes manipulate mate choice
Sex chromosomes manipulate mate choice