- OUTLOOK
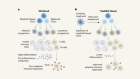
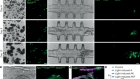
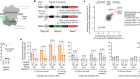
The biological clean-ups that could combat age-related disease
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 601, S15-S17 (2022)
doi: https://doi.org/10.1038/d41586-022-00075-w
This article is part of Nature Outlook: Ageing, an editorially independent supplement produced with the financial support of third parties. About this content.
References
Fernández, Á. F. et al. Nature 558, 136–140 (2018).
Wang, C. et al. Autophagy 8, 1–14 (2021).
Rocchi, A. et al. PLoS Genet. 13, e1006962 (2017).
Shi, M. et al. FASEB J. 34, 3129–3150 (2020).
Anguiano, J. et al. Nature Chem. Biol. 9, 374–382 (2013).
Bourdenx, M. et al. Cell 184, 2696–2714 (2021).
Dong, S. et al. Nature 591, 117–123 (2021).
Xie, C. et al. Nature Biomed. Eng. https://doi.org/10.1038/s41551-021-00819-5 (2022).
Siddiqi, F. H. et al. Nature Commun. 10, 1817 (2019).
Takahashi, D. et al. Mol. Cell 76, 797–810 (2019).
Li, Z. et al. Nature 575, 203–209 (2019).

 Ageing
Ageing
 Does the human lifespan have a limit?
Does the human lifespan have a limit?
 How the COVID-19 pandemic might age us
How the COVID-19 pandemic might age us
 Robots rise to meet the challenge of caring for old people
Robots rise to meet the challenge of caring for old people
 Support for LGBTQ+ people in later life
Support for LGBTQ+ people in later life
 Tackling the crisis of care for older people: lessons from India and Japan
Tackling the crisis of care for older people: lessons from India and Japan
 Research round-up: Ageing
Research round-up: Ageing
 Turning back time with epigenetic clocks
Turning back time with epigenetic clocks