- NEWS
- Clarification 09 October 2021
How antiviral pill molnupiravir shot ahead in the COVID drug hunt
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-021-02783-1
Updates & Corrections
-
Clarification 09 October 2021: This story was updated to clarify that the two Indian drugmakers testing a generic version of molnupiravir did so independently of Merck, and to add comments from Merck indicating that moderate cases of COVID-19 in India are defined as being more severe than those in the United States.
References
Beigel, J. H. et al. N. Engl. J. Med. 383, 1813-1826 (2020).
Sheahan, T. P. et al. Sci. Transl. Med. 12, eabb5883 (2020).
Cox, R. M. et al. Nature Microbiol. 6, 11-18 (2021).

 The race for antiviral drugs to beat COVID — and the next pandemic
The race for antiviral drugs to beat COVID — and the next pandemic
 Hopes rise for coronavirus drug remdesivir
Hopes rise for coronavirus drug remdesivir
 Coronavirus breakthrough: dexamethasone is first drug shown to save lives
Coronavirus breakthrough: dexamethasone is first drug shown to save lives
 Dozens of coronavirus drugs are in development — what happens next?
Dozens of coronavirus drugs are in development — what happens next?
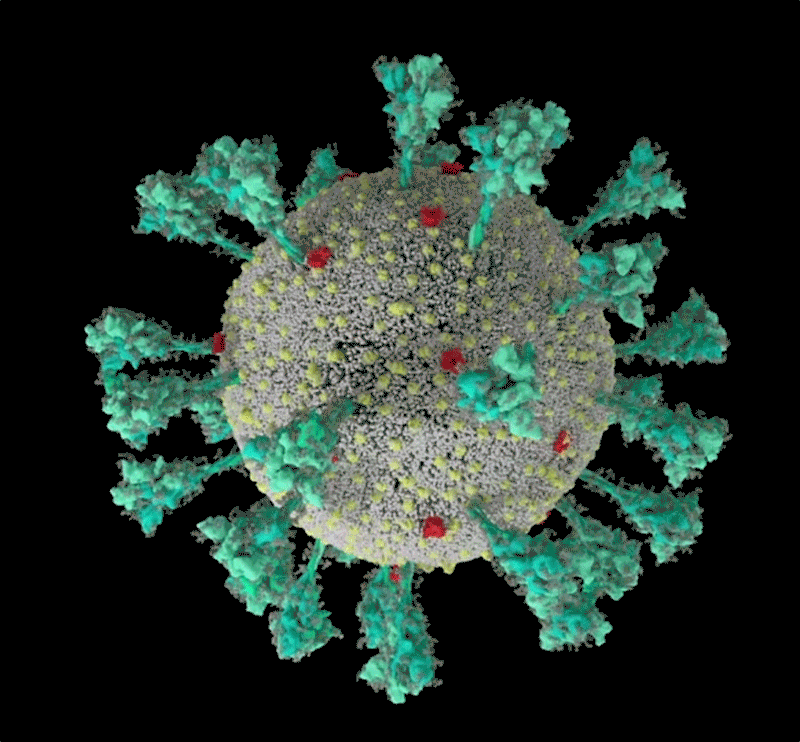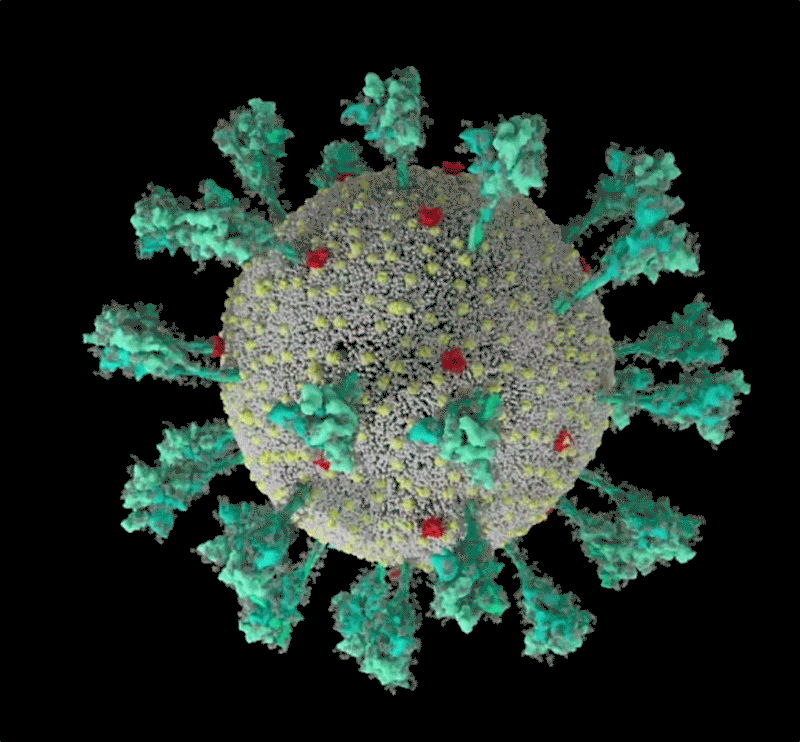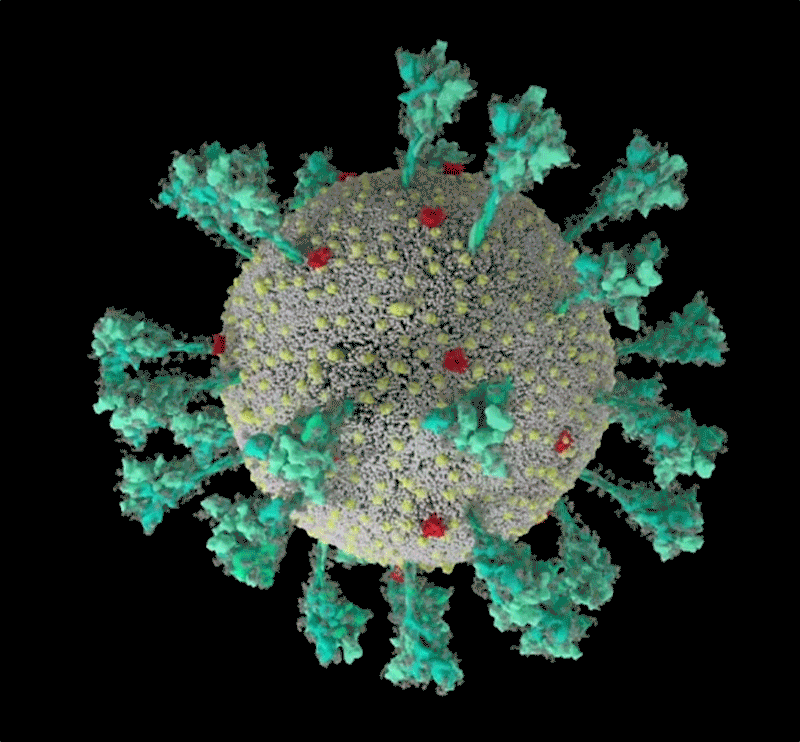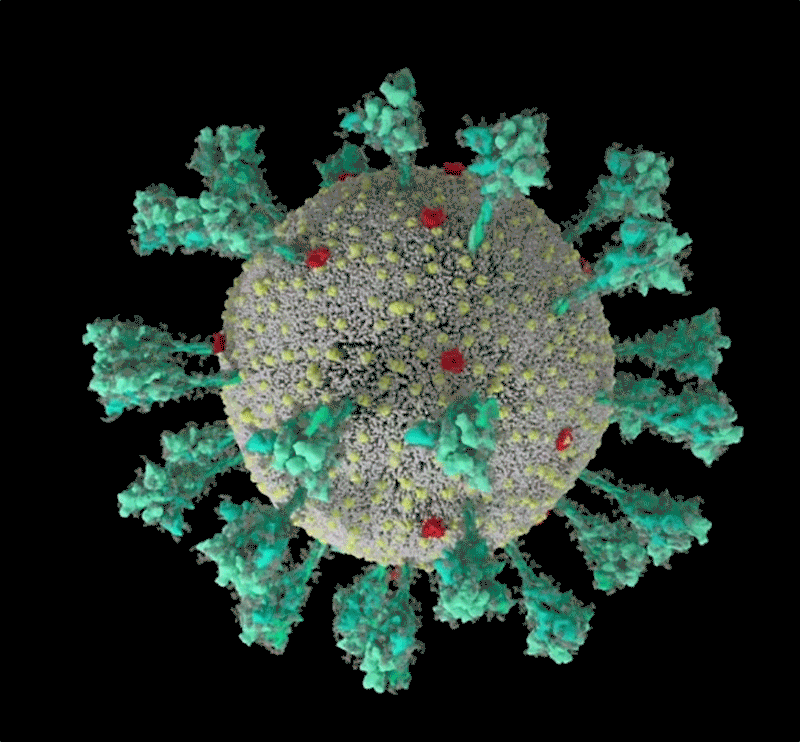 How the coronavirus infects cells — and why Delta is so dangerous
How the coronavirus infects cells — and why Delta is so dangerous