- NEWS AND VIEWS
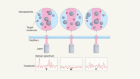
Ion dynamics in battery materials imaged rapidly
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 594, 503-504 (2021)
doi: https://doi.org/10.1038/d41586-021-01600-z
References
Merryweather, A. J., Schnedermann, C., Jacquet, Q., Grey, C. P. & Rao, A. Nature 594, 522–528 (2021).
Klett, M. et al. J. Am. Chem. Soc. 134, 14654–14657 (2012).
Ebner, M., Marone, F., Stampanoni, M. & Wood, V. Science 342, 716–720 (2013).
Ilott, A. J., Mohammadi, M., Chang, H. J., Grey, C. P. & Jerschow, A. Proc. Natl Acad. Sci. USA 113, 10779–10784 (2016).
Yermukhambetova, A. et al. Sci. Rep. 6, 35291 (2016).
Song, B. et al. ACS Energy Lett. 4, 2402–2408 (2019).
Steinruck, H.-G. et al. Energy Environ. Sci. 13, 4312–4321 (2020).
Gao, T. et al. Joule 5, 393–414 (2021).
Ortega-Arroyo, J. & Kukura, P. Phys. Chem. Chem. Phys. 14, 15625–15636 (2012).
Fraggedakis, D. et al. Energy Environ. Sci. 13, 2142–2152 (2020).
Mistry, A., Usseglio-Viretta, F. L. E., Colclasure, A., Smith, K. & Mukherjee, P. P. J. Electrochem. Soc. 167, 090542 (2020).
Mistry, A., Franco, A. A., Cooper, S. J., Roberts, S. A. & Viswanathan, V. ACS Energy Letters 6, 1422–1431 (2021).
Competing Interests
The author declares no competing interests.

 Read the paper: Operando optical tracking of single-particle ion dynamics in batteries
Read the paper: Operando optical tracking of single-particle ion dynamics in batteries
 Machine-learning techniques used to accurately predict battery life
Machine-learning techniques used to accurately predict battery life
 Long-lived electrodes for plastic batteries
Long-lived electrodes for plastic batteries