- NEWS AND VIEWS
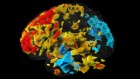
Attraction and repulsion cooperate during brain-circuit wiring
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 594, 341-343 (2021)
doi: https://doi.org/10.1038/d41586-021-01502-0
References
Sperry, R. W. Proc. Natl Acad. Sci. USA 50, 703–710 (1963).
Sanes, J. R. & Zipursky, S. L. Cell 181, 536–556 (2020).
Pederick, D. T. et al. Science https://doi.org/10.1126/science.abg1774 (2021).
Igarashi, K. M., Ito, H. T., Moser, E. I. & Moser, M.-B. FEBS Lett. 588, 2470–2476 (2014).
Berns, D. S., DeNardo, L. A., Pederick, D. T. & Luo, L. Nature 554, 328–333 (2018).
Del Toro, D. et al. Cell 180, 323–339e.19 (2020).
Sando, R., Jiang, X. & Südhof, T. C. Science 363, eaav7969 (2019).
Competing Interests
C.H. is in the process of transitioning from his appointment at Harvard Medical School to UCSF where he will also be a Chan Zuckerberg Biohub investigator. Stephen Quake is a co-author of the study referred to in this News & Views article and is currently co-president of the Chan Zuckerberg Biohub.

 Maternal microbes support fetal brain wiring
Maternal microbes support fetal brain wiring
 A bitter–sweet symphony
A bitter–sweet symphony