- NEWS AND VIEWS
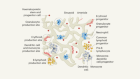
Cancer stem cells in the gut have a bad influence on neighbouring cells
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 594, 340-341 (2021)
doi: https://doi.org/10.1038/d41586-021-01379-z
References
Yum, M. K. et al. Nature 594, 442–447 (2021).
van Neerven, S. M. et al. Nature 594, 436–441 (2021).
Flanagan, D. J. et al. Nature 594, 430–435 (2021).
Kinzler, K. W. & Vogelstein, B. Cell 87, 159–170 (1996).
Kwong, L. N. & Dove, W. F. Adv. Exp. Med. Biol. 656, 85–106 (2009).
Suijkerbuijk, S. J. E., Kolahgar, G., Kucinski, I. & Piddini, E. Curr. Biol. 26, 428–438 (2016).
Galluzzi, L., Spranger, S., Fuchs, E. & López-Soto, A. Trends Cell Biol. 29, 44–65 (2019).
Welner, R. S. et al. Cancer Cell 27, 671–681 (2015).
Reynaud, D. et al. Cancer Cell 20, 661–673 (2011).
Sancho, M. et al. Dev. Cell 26, 19–30 (2013).
Liu, N. et al. Nature 568, 344–350 (2019).
Tanimura, N. & Fujita, Y. Semin. Cancer Biol. 63, 44–48 (2020).
Merino, M. M. et al. Cell 160, 461–476 (2015).
Pentinmikko, N. et al. Nature 571, 398–402 (2019).
Kakiuchi, N. & Ogawa, S. Nature Rev. Cancer 21, 239–256 (2021).
Competing Interests
The authors declare no competing interests.

 Read the paper: Tracing oncogene-driven remodelling of the intestinal stem cell niche
Read the paper: Tracing oncogene-driven remodelling of the intestinal stem cell niche
 Read the paper: Apc-mutant cells act as supercompetitors in intestinal tumour initiation
Read the paper: Apc-mutant cells act as supercompetitors in intestinal tumour initiation
 Read the paper: NOTUM from Apc-mutant cells biases clonal competition to initiate cancer
Read the paper: NOTUM from Apc-mutant cells biases clonal competition to initiate cancer
 A gut punch fights cancer and infection
A gut punch fights cancer and infection
 A mutational signature that can be made by a bacterium arises in human colon cancer
A mutational signature that can be made by a bacterium arises in human colon cancer