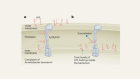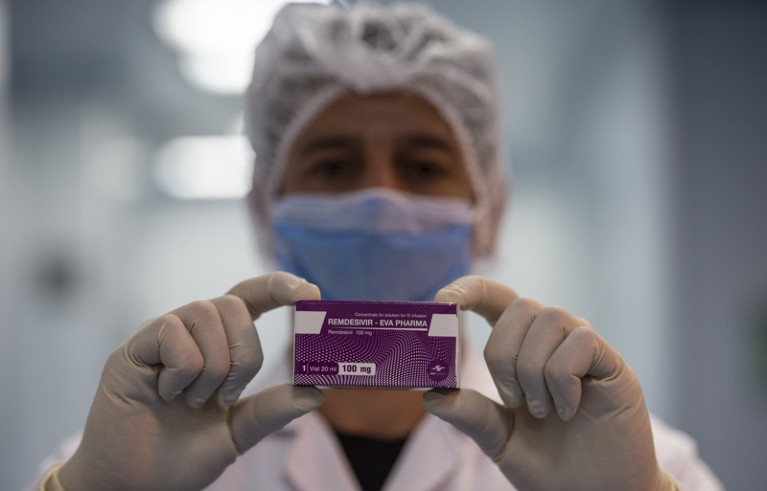
Remesivir should not have been the only ‘shovel-ready’ antiviral drug for potential COVID-19 treatment.Credit: Mohamed Hossam/EPA-EFE/Shutterstock
The global disruption caused by COVID-19 has been a shock, but not a surprise. For years, researchers have warned that a deadly viral pandemic could bring nations to their knees. They urged governments and pharmaceutical companies to work together on broad-spectrum antiviral drugs — capable of beating a variety of viruses — and to ensure that those drugs were ready for testing in humans when disaster struck.
Influenza viruses, coronaviruses and relatives of Ebola were all considered potential threats. But when the COVID-19 pandemic hit, the medicine cabinet was all but empty. Remdesivir was one of just a few ‘shovel ready’ antiviral drugs that researchers could quickly put into human testing. In early tests, it showed some success in reducing the time that people with COVID-19 spent in hospital. But other studies have not shown the drug to be beneficial.
Coronavirus vaccines are rightly being celebrated, but antiviral drugs could — and would — have had a crucial, life-saving role. The public sector should have rallied quickly to develop them, as it did for vaccines, but this has not yet happened. Although scientists and companies are starting to make concerted efforts, most governments are not treating this issue with the same urgency as they have vaccines. Unless that changes, the world might remain just as poorly prepared for the next viral pandemic.
The race for antiviral drugs to beat COVID — and the next pandemic
The warnings — in the past 20 years alone — have been loud and clear. An outbreak of severe acute respiratory syndrome (SARS) in 2003 prompted calls for more antiviral drug development. But there was little action by funders, partly because the threat subsided. Another warning came a decade later, after an outbreak of Middle East respiratory syndrome (MERS). Again, governments and industry paid little heed. Some drug programmes trundled on, but without proper investment towards a clear goal — the production of drugs that have been safety-tested in people and that could be made ready for fast and decisive clinical trials.
This pandemic could change that. As reported in a News Feature this week, a number of initiatives are under way to right this wrong. The COVID R&D Alliance, a consortium of more than 20 life-science companies and venture-capital firms from around the world, is aiming to create an organization that will accelerate the development of drugs against coronaviruses. The consortium, which was set up last year, plans to prepare 25 candidate medicines for trials in humans, so that at least some can be ready for larger trials when the next pandemic-causing virus strikes.
The COVID R&D Alliance, and another global project called the Rapidly Emerging Antiviral Drug Development Initiative, are in the process of raising funds from industry and governments. The US National Institutes of Health (NIH) is planning to invest heavily in creating drugs to fight SARS-CoV-2. It is essential that the agency is given the funding to make strategic bets in creating drugs for the next pandemic.
The antibiotic paradox: why companies can’t afford to create life-saving drugs
In contrast to these efforts, vaccine development happened at lightning speed when the richest countries agreed to provide funding while vaccines were being developed. Some countries agreed to purchase the resulting vaccines, even if they failed. A similar funding vehicle — one based on both the public and private sectors being willing to take risks — must be considered for antiviral drugs.
There are models for success here. Remdesivir was almost ready to be tested, thanks to the work of researchers backed by an NIH project called the Antiviral Drug Discovery and Development Center. This was launched in 2014 to screen drug libraries for candidates that could inhibit viruses, including influenza and coronaviruses. Remdesivir’s effectiveness in animal models was established in 2017. It was tested in people and shown to be safe during an Ebola outbreak in the Democratic Republic of the Congo, Liberia and Guinea. That meant the drug was ready for more widespread human testing should the need arise.
At least twice before, world leaders were warned to build up a medicine chest of ready antiviral drugs. But momentum fizzled out as previous outbreaks ended, and because of a perennial argument between governments and industry over who should contribute what share of the bill.
The pandemic has shown that this was wrong-headed. Public health requires investments in drugs to counter any pathogen with epidemic or pandemic potential; that includes many airborne and mosquito-borne diseases, and, of course, the threat of antibiotic resistance remains. Governments collectively provided around US$90 billion in funding for vaccines in 2020, and some of the work this has funded will help in future pandemics. They must do the same for antiviral drugs, which need to be distributed equitably. The world cannot afford to be caught with an empty cabinet again.

 The race for antiviral drugs to beat COVID — and the next pandemic
The race for antiviral drugs to beat COVID — and the next pandemic
 The antibiotic paradox: why companies can’t afford to create life-saving drugs
The antibiotic paradox: why companies can’t afford to create life-saving drugs
 What it will take to vaccinate the world against COVID-19
What it will take to vaccinate the world against COVID-19