- NEWS AND VIEWS
Repeat DNA expands our understanding of autism spectrum disorder
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 589, 200-202 (2021)
doi: https://doi.org/10.1038/d41586-020-03658-7
References
Hannan, A. J. Nature Rev. Genet. 19, 286–298 (2018).
Mitra, I. et al. Nature 589, 246–250 (2021).
Schaaf, C. P. et al. Nature Rev. Genet. 21, 367–376 (2020).
Trost, B. et al. Nature 586, 80–86 (2020).
Dolzhenko, E. et al. Genome Biol. 21, 102 (2020).
Eichler, E. E. et al. Nature Rev. Genet. 11, 446–450 (2010).
Hannan, A. J. Trends Genet. 26, 59–65 (2010).
Breuss, M. W. et al. Nature Med. 26, 143–150 (2020).
Mousavi, N. et al. Bioinformatics https://doi.org/10.1093/bioinformatics/btaa736 (2020).
Nakamori, M. et al. Nature Genet. 52, 146–159 (2020).

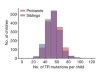 Read the paper: Patterns of de novo tandem repeat mutations and their role in autism
Read the paper: Patterns of de novo tandem repeat mutations and their role in autism
 Mum’s bacteria linked to baby’s behaviour
Mum’s bacteria linked to baby’s behaviour
 Untangling autism
Untangling autism






