A key part of tackling COVID-19 is understanding why some people experience more-severe symptoms than do others. Earlier this year, a segment of DNA 50,000 nucleotides long (corresponding to 0.002% of the human genome) was found to have a strong association with severe COVID-19 infection and hospitalization1. Writing in Nature, Zeberg and Pääbo2 report that this region is inherited from Neanderthals. Their results not only shed light on one reason that some people are more susceptible to severe disease, but also provide insights into human evolutionary biology.
Read the paper: The major genetic risk factor for severe COVID-19 is inherited from Neanderthals
DNA sequences that are physically close to one other in the genome are often inherited (linked) together. These blocks of DNA, known as haplotypes, therefore contain tightly linked variants — DNA sequences or nucleotides that vary between individuals in a population. For example, the COVID-19 risk haplotype described earlier this year1 harbours variants across its entire 50,000-nucleotide span that are inherited together more than 98% of the time. Long haplotypes such as this could be a result of positive selection, maintained in our genomes because they contributed to our species’ chances of survival and reproductive success. They could also be introduced as a result of interbreeding with archaic hominin species such as the Denisovans and Neanderthals.
Some 1–4% of the modern human genome comes from these ancient relatives3. Many of the surviving archaic genes are harmful to modern humans, and are associated with infertility and an increased risk of disease4. But a few are beneficial. Examples include the Denisovan-like version of a gene called EPAS1 that helps modern Tibetans to cope with life at extremely high altitudes5, a Neanderthal gene that increases our sensitivity to pain6 and others that help us fend off viruses7.
To investigate whether the COVID-19 risk haplotype might have been introduced from our ancient relatives, Zeberg and Pääbo compared the region with an online database of archaic genomes from around the world. They found the region to be closely related to that in the genome of a Neanderthal individual that lived in modern-day Croatia around 50,000 years ago, but it was not related to any known Denisovan genomes.
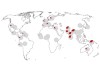
The authors next checked the prevalence of the Neanderthal-derived haplotype in the modern human population. They report that it is rare or completely absent in east Asians and Africans. Among Latin Americans and Europeans, the risk haplotype is maintained at a modest frequency (4% and 8%, respectively). By contrast, the haplotype occurs at a frequency of 30% in individuals who have south Asian ancestry, reaching as high as 37% in those with Bangladeshi heritage (Fig. 1).
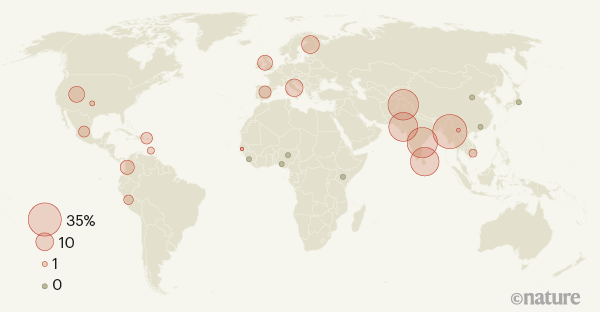
Figure 1 | Uneven global spread of a genetic risk factor for COVID-19. Zeberg and Pääbo2 report that a long sequence of DNA that is associated with severe COVID-19 infection and hospitalization is derived from Neanderthals. The sequence is unevenly distributed across modern human populations. This map shows the frequency at which the risk factor is found in various populations from around the world. The sequencing data for these populations were gathered by the 1000 Genomes Project10. (Adapted from Fig. 3 of ref. 2.)
The researchers therefore speculate that the Neanderthal-derived haplotype is a substantial contributor to COVID-19 risk in specific groups. Their hypothesis is supported by hospital data8 from the Office for National Statistics in the United Kingdom, which indicates that individuals of Bangladeshi origin in the country are twice as likely to die from COVID-19 as are members of the general population (although other risk factors will, of course, contribute to these statistics).
Why has this haplotype been retained in some populations? The authors posit that it might be protective against other ancient pathogens, and therefore positively selected for in certain populations around the world9. But when individuals are infected with the SARS-CoV-2 coronavirus, the protective immune response mediated by these ancient genes might be overly aggressive, leading to the potentially fatal immune response observed in people who develop severe COVID-19 symptoms. As a result, a haplotype that at times in our past might have been beneficial for survival could now be having an adverse effect.
A race to determine what drives COVID-19 severity
Despite the correlation between this risk haplotype and clinical outcomes, genetics alone do not determine a person’s risk of developing severe COVID-19. Our genes and their origins clearly influence the development and progression of COVID-19 (and other infectious diseases), but environmental factors also have key roles in disease outcomes.
For example, although the Neanderthal-derived risk haplotype is almost completely absent in people with African ancestry, this population has a higher COVID-19 mortality rate than do people of other ethnic backgrounds, even after adjusting for geography and socio-economic factors (see go.nature.com/3jcxezx (‘Demographics’ tab) and go.nature.com/2h4qfqu, for example). Social inequality and its repercussions seem likely to account for a larger proportion of the risk of COVID-19 death than does Neanderthal-derived DNA.
It is fascinating to think that our ancestor’s genetic legacy might be playing a part in the current pandemic. However, the underlying impact of the inherited DNA on the body’s response to the virus is unclear. Ongoing global efforts to study associations between our genetics and COVID-19 by analysing more individuals from diverse populations, such as that being undertaken by the COVID-19 Host Genetics Initiative (www.covid19hg.org), will help us to develop a better understanding of the disease’s aetiology. It is important to acknowledge that, although genes involved in the COVID-19 response might be inherited, social factors and behaviours (such as social distancing and mask wearing) are in our control, and can effectively reduce the risk of infection.

 Read the paper: The major genetic risk factor for severe COVID-19 is inherited from Neanderthals
Read the paper: The major genetic risk factor for severe COVID-19 is inherited from Neanderthals
 A race to determine what drives COVID-19 severity
A race to determine what drives COVID-19 severity
 On the origin of our species
On the origin of our species