
Intensive-care units need more treatment options for people with COVID-19.Credit: Natacha Pisarenko/AP/Shutterstock
The race is on to develop a vaccine to protect people against the new coronavirus, SARS-CoV-2. Less than a year after the virus was identified, almost 200 vaccines are in development and more than 40 are in clinical trials — thanks, in part, to an unprecedented collaborative effort by researchers around the world. The vaccine quest makes it necessary for researchers to answer questions about how the body’s immune system responds to the virus, and why some people experience severe symptoms, whereas others recover quickly.
As we explain in this second instalment of Nature’s series of coronavirus progress reports, a safe and effective vaccine represents the most powerful weapon against the virus, at both the individual and the population level — but it will not be the only one. Mask-wearing, hand-washing and social distancing will need to continue for some time.
The immunity challenge
At the start of the coronavirus pandemic, researchers lacked an understanding of how natural immunity to SARS-CoV-2 might develop. But that quickly changed. Within months, studies had shown that infected people can make antibodies that neutralize the virus1, as well as T cells that can recognize and kill SARS-CoV-2-infected cells2.
All eyes on a hurdle race for a SARS-CoV-2 vaccine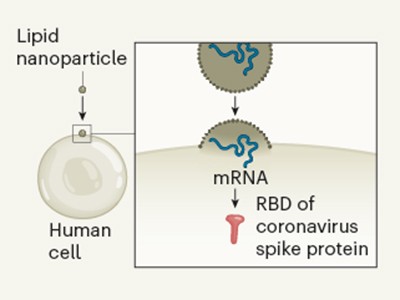
Further research has revealed that even people who have not been infected with the virus can have antibodies3 and T cells2 that recognize SARS-CoV-2. This might be the result of people having previously been infected with related coronaviruses that cause the common cold. However, it isn’t yet known whether these pre-existing responses provide any protection against infection with SARS-CoV-2.
The presence of these antibodies and T-cell responses was promising news for vaccine development; if natural infection can elicit these sorts of responses, then vaccination might be able to elicit similar or more potent protective responses.
Immunity has implications for therapies, too
In August, the US Food and Drug Administration (FDA) gave emergency authorization to a treatment called convalescent plasma. This treatment involves giving people with COVID-19 blood plasma from individuals recovering from the disease, the rationale being that the plasma contains many antibodies that recognize SARS-CoV-2. However, the consensus among clinicians and researchers is that the FDA’s decision was premature, because not all these antibodies will be protective.
There is not yet definitive evidence that this treatment is effective4, although trials are ongoing to explore whether convalescent plasma from people with high levels of neutralizing antibodies against the virus might be beneficial in the treatment of severe COVID-19.
A more promising approach than convalescent plasma would be to isolate specific and potent neutralizing antibodies from infected patients, and to develop antibody treatments based on these. This approach has been successful for other viral diseases: just last week, the FDA approved one such treatment for Ebola virus. And trials are under way to test the effectiveness of such monoclonal antibodies against COVID-19.
How likely is reinfection?
Another urgent question is whether a person who has recovered from COVID-19 is protected from future infection. August saw the first confirmed report of a person being reinfected after they had recovered from COVID-19; this was determined by sequencing of the viral strains responsible for the original and the second infections5. There have since been a handful of other confirmed cases of coronavirus reinfection6, although against a background of more than 40 million infections globally.
People who recover from other types of coronavirus infection can be reinfected, because the antibody responses in those cases are relatively short-lived7. In the case of COVID-19, it is not yet known how long any potential protection might last; how common reinfection is; or to what extent reinfected individuals are going undetected. In at least two of the documented cases of reinfection, the disease was more severe the second time around6, although mild cases of reinfection might go undetected.
Autopsy studies indicate that the germinal centres — structures in the lymph nodes and spleen where antibody-producing B cells mature and differentiate — can be absent in people with severe COVID-19, causing a loss of antibody-producing B cells8. This might mean that the antibody response to natural infection is not long-lasting.
But other studies on infected people have shown evidence of more durable responses: antibodies peak soon after infection and then wane after the infection has been cleared, remaining at lower levels thereafter, at least for the time frame studied9.
How does the immune response affect disease severity?
One notable feature of severe COVID-19 is that the health of affected people often seems to deteriorate rapidly during the second week of infection, in many cases after initially relatively mild symptoms. Early studies of these patients hinted at an imbalanced immune response, with an overactive inflammatory response failing to control the virus and instead driving the disease10.
The drug dexamethasone, which dampens the inflammatory response and is widely used to treat many inflammatory conditions, has been one of the few therapies that has so far been proven11 to reduce mortality from severe COVID-19.
Work published last month showed that at least 10% of men and a smaller percentage of women with severe disease have auto-antibodies12. These interfere with the function of a group of proteins known as interferons — a crucial component of the anti-viral immune response.
Vaccines: the questions that need answers
As the effort to develop vaccines gathers pace, key questions will need to be answered to ensure that they are safe and effective.
Read the paper: SARS-CoV-2 vaccines in development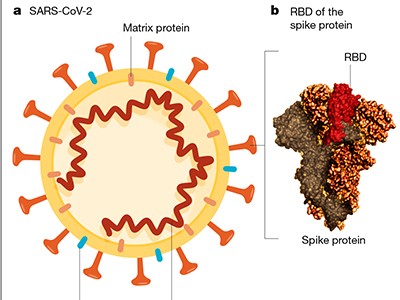
On the plus side, the virus doesn’t seem to be mutating fast — unlike, for example, the influenza virus. This means that SARS-CoV-2 probably won’t quickly mutate to evade a vaccine-induced response. At the same time, it is not yet known whether vaccine-induced immunity will be short- or long-lived, nor how effective a vaccine will be in older people, whose immune systems often respond less well to vaccination. If immunity is short-lived, then vaccinated populations will need regular boosters.
A host of logistical and supply-chain questions will take time to resolve, too. The administration of vaccines requires equipment such as vials and needles. In some countries, stocks might need to be kept in cold storage, and in many places, extra health workers will need to be recruited and trained. Then there is the question of which countries — and which groups within countries — should get priority access. Several organizations are working to resolve these questions.
Trust and verify
COVID-19 vaccines will be considered for approval by the World Health Organization (WHO) under its emergency-use listing — in which a vaccine is approved for use while trials are still taking place. The WHO and national regulators are working under tremendous pressure from governments and the pharmaceutical industry, but all sides must realize that there can be no short cuts to regulatory approval. Public trust in vaccines is essential, which is why regulators must be allowed to complete their work without interference.
Vaccine hesitancy presents further challenges. Any new vaccine must be carefully monitored for adverse effects, especially in vulnerable populations. As we have written before, overcoming vaccine hesitancy will also require radical transparency from drug companies and their academic partners.
Much of the coronavirus vaccine effort is an example of just what can be achieved when researchers, clinicians, funders, regulators, corporations — in short, people — come together to act in the common good. A working vaccine is essential, but it must be safe and effective, and it needs to be distributed equitably and to those who need it most. Until it arrives, and probably for a long time afterwards, people must stick to solutions that work — rigorous testing, tracing and isolating — and change their behaviour to help curb the virus’s spread.


 All eyes on a hurdle race for a SARS-CoV-2 vaccine
All eyes on a hurdle race for a SARS-CoV-2 vaccine
 Face masks: what the data say
Face masks: what the data say
 Read the paper: SARS-CoV-2 vaccines in development
Read the paper: SARS-CoV-2 vaccines in development
 The unequal scramble for coronavirus vaccines — by the numbers
The unequal scramble for coronavirus vaccines — by the numbers
 Six months of coronavirus: the mysteries scientists are still racing to solve
Six months of coronavirus: the mysteries scientists are still racing to solve
 Progress report on the coronavirus pandemic
Progress report on the coronavirus pandemic






