Last month, a grand experiment was launched. Its aim? To speed up the development of COVID‑19 vaccines and make sure they are distributed equitably among higher- and lower-income countries.
This welcome endeavour is called the COVID-19 Vaccine Global Access (COVAX) initiative. It is co-led by the World Health Organization (WHO), the Coalition for Epidemic Preparedness Innovations (CEPI) and Gavi, the Vaccine Alliance. As of 1 October, 167 countries have signed up, covering nearly two-thirds of the global population. More have expressed interest, according to Gavi.
The initiative covers several vaccines currently in testing. It aims to ensure access to whichever ones prove to be effective. Under this scheme, even poor nations should have enough vaccines to protect health-care workers and the most vulnerable 20% of their populations.
Still, Africa has reasons to worry. Already, several high-income countries have signed their own contracts with individual companies to buy selected vaccines. The United States, for example, has made deals worth upwards of US$6 billion with several firms. An analysis by the international charity Oxfam finds that, even if all five of the most-advanced vaccine candidates succeed, there will not be enough vaccine for most of the world’s people until 2022.
The unequal scramble for coronavirus vaccines — by the numbers
We’ve seen a scramble for access to therapies before. It happened with HIV and H5N1 influenza, for example. And Africa has ended up at the end of the queue every time. Yet the global economy depends on the continent for its exports of raw materials, food, energy and labour.
This experience — and the fact that other infectious diseases will surely emerge — is why Africa needs a coordinated strategy to develop, finance, manufacture and deliver vaccines across the continent. For the past few months, the Africa Centres for Disease Control and Prevention (Africa CDC) in Addis Ababa, where we work, has been developing this, with leaders from the African Union and in global health.
Here we describe what must be done.
Learn from history
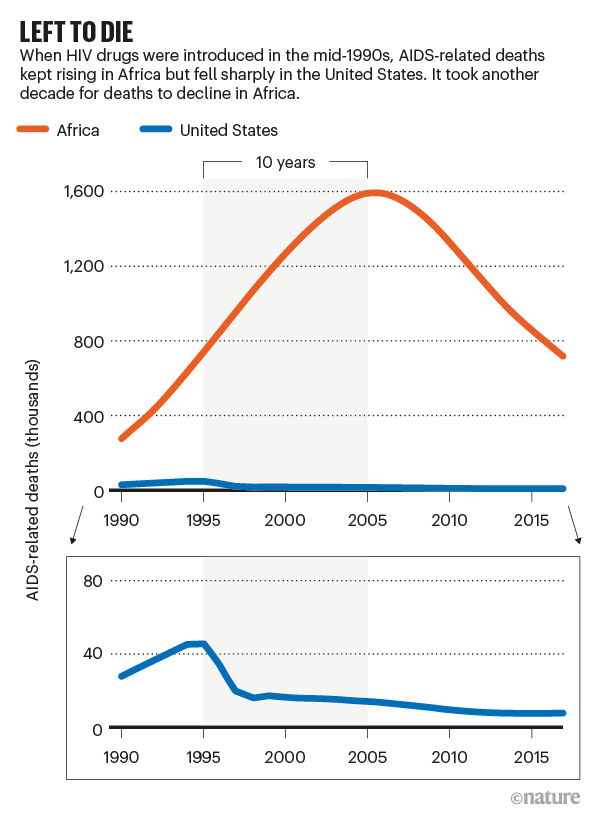
Antiretroviral drugs to treat HIV entered the market in the mid-1990s. At the time, one of us (J.N.) was working in Côte d’Ivoire as part of a US Centers for Disease Control and Prevention project that was struggling to combat HIV in the country without access to medicine. The prices that companies set for these drugs put them out of reach. As deaths in rich countries plummeted, infected people were left to die across Africa (see ‘Left to die’). It is estimated that, between 1997 and 2007, 12 million Africans died waiting for enough life-saving drugs to reach the continent. The drugs’ arrival was largely thanks to the efforts of the US President’s Emergency Plan For AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Sources: UNAIDS/US CDC
In 2004, the highly pathogenic avian influenza virus H5N1 re-emerged, prompting fears of an overwhelming, global pandemic1. Negotiations by the WHO to share and stockpile doses of an eventual vaccine broke down. At one point, tensions were so high that Indonesia refused to share H5N1 virus samples that were crucial for surveillance. Five years later, another strain of pandemic flu (H1N1) emerged, and rich countries placed large pre-orders of vaccine, buying almost all of the doses that could possibly be manufactured. Many of these countries promised to donate vaccines under plans sponsored by the WHO and the United Nations. They then reneged or otherwise moved to secure their own countries’ supply above that of others.
Earlier this year, Africa was elbowed out of the diagnostics market for SARS-CoV-2 — although the situation is now improving. Shortages of materials were initially the greatest limitation in fighting the pandemic on the continent2. So, in April, Africa CDC launched a Partnership to Accelerate COVID-19 Testing (PACT). Training was set up, and technicians in Africa have now conducted more than 14.5 million COVID-19 tests. Countries including Kenya, Ethiopia, Nigeria, Morocco, Senegal and South Africa have started making test kits. At the time of writing, Ethiopia is on track to produce some 10 million COVID-19 PCR-based test kits per year for use across Africa. This is far below its needs, but many more than seemed possible when we launched PACT.
Cost and scale
The COVAX initiative promises to benefit Africa, and many people on other continents. It hopes to offer lower-income countries equitable access to a diverse portfolio of potential vaccines at highly subsidized prices. This international cooperation and solidarity is laudable and essential.
So is preparation across the whole of Africa for testing, purchasing and delivery. Africa, like all continents, needs an accessible vaccine to save the lives and health of vulnerable populations, and to maintain economic development. As of 1 October, more than 1.4 million people were known to have been infected with SARS-CoV-2 in Africa, with more than 36,000 deaths. As COVID-19 continues to spread, thousands more lives will be lost. The World Bank estimates that economic growth in sub-Saharan Africa will decline from 2.4% in 2019 to between 2.1 and −5.1% in 2020, the first recession in the region in 25 years. In addition to the coronavirus pandemic, many nations in Africa are reeling from the worst locust plague in 50 years, and a terrible drought is predicted to occur in East Africa. A COVID-19 vaccine could at least help to mitigate this dire situation.

A woman wearing a face mask walks past a COVID-19 mural in Nairobi.Credit: Tony Karumba/AFP/Getty
To vaccinate 60% of its population (the estimated minimum requirement for herd immunity3), Africa will need about 1.5 billion doses of vaccine. (Its population is 1.2 billion, and most vaccine candidates require two doses.) The cost of the vaccine and of building systems and structures required for delivery is estimated at between $7 billion and $10 billion, according to Africa CDC. For comparison, the 2020 US PEPFAR budget was $6.9 billion.
This level of vaccination far exceeds that in the successful system that immunized young children against a suite of diseases, at least until it was disrupted by the pandemic. These include tuberculosis, diphtheria, tetanus, whooping cough, poliomyelitis, measles, hepatitis B, yellow fever and the bacterial infection Haemophilus influenzae (which can cause severe infections of the blood or lining of the brain and spinal cord). The WHO’s Expanded Program of Immunization has delivered millions of vaccine doses to children in Africa since 1977. Nothing on this scale has ever been developed to reach adults.
A ‘whole of Africa’ coordinated approach is needed to prepare for the development, purchase, access and roll-out of a COVID‑19 vaccine. That must be built now. Success will require collaboration between political leaders on the continent and those elsewhere, including the WHO, Gavi, CEPI, regulatory agencies, implementing partners, donors and the private sector.
Let Africa into the market for COVID-19 diagnostics
In June, the African Union Commission and Africa CDC convened a virtual conference. More than 3,000 political leaders and technical experts discussed COVID-19 vaccine needs and a continental strategy. In August, the African Union Bureau of Heads of State and Government endorsed the strategy put forward by Africa CDC, reiterating how past experience in global health shows that Africa must move decisively, effectively and collectively to secure access to vaccines and life-saving therapy.
Three pillars
The strategy has three pillars. The first is to accelerate African involvement in the clinical development of a vaccine. The second is to ensure that Africa can access a sufficient share of the global supply. The third is to remove barriers to widespread delivery and uptake of the vaccine across Africa.
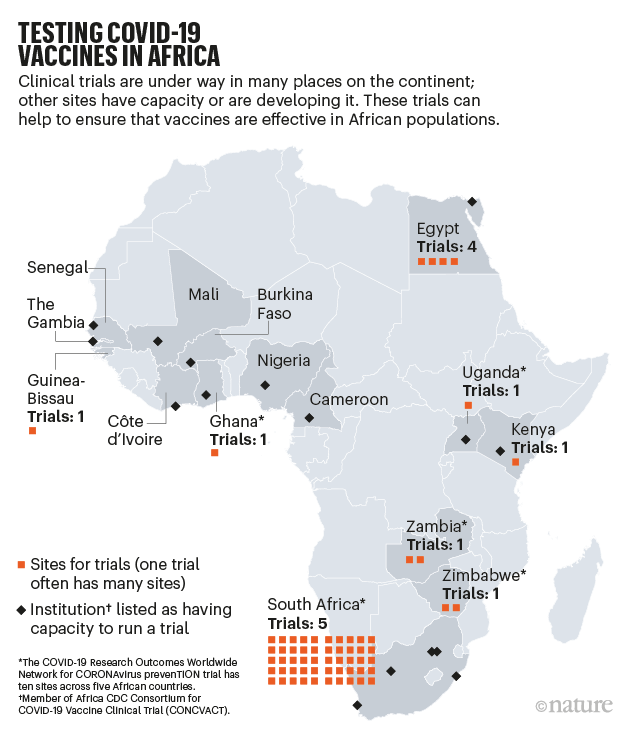
Trials in Africa. The best way to make sure vaccines are safe and effective for an African population is to test them in Africa, which has a long history of participating in clinical trials. In July this year, Africa CDC set up the Consortium for COVID-19 Vaccine Clinical Trial (CONCVACT). It has inventoried sites that can test vaccines in humans (see ‘Testing COVID-19 vaccines in Africa’ and Supplementary information) and is working to set up more infrastructure, such as arranging more training in international standards, including good clinical practice and establishing independent review boards. CONCVACT is facilitating partnerships and coordination between vaccine developers, African clinical-trial sites, sponsors and funders, including the WHO and the European and Developing Countries Clinical Trials Partnership.
Source: Africa CDC
Funds and factories. New financing will be required to pay the billions of dollars for COVID‑19 vaccinations, including advance payments to secure supply. The Africa Export Import Bank (Afreximbank) has committed to a vaccine-financing framework for Africa. This will allow pooled purchases of medical supplies and support for vaccine manufacture. Countries will issue promissory notes to Afreximbank, which will arrange about $4 billion in revolving credit to back orders from African suppliers that have been certified by Africa CDC.
If a COVID‑19 vaccine needs to be administered yearly, importing it might not be reliable or feasible, especially if the vaccine is liquid (and thus heavy) or requires cold storage. Vaccine-manufacturing capacity in Africa is nascent, but real. Senegal and South Africa already produce vaccines for diseases such as yellow fever and tuberculosis. Although these are made by different technologies from those likely to be used for COVID-19, the mindset and training required are similar. GALVMed, an international organization focused on livestock vaccines, with offices in Kenya, India and the United Kingdom, might also be able to pivot to make human-grade vaccines. We have so far identified eight companies or organizations in several African countries that can reasonably aspire to retool their operations to manufacture COVID-19 vaccines. We are working with them to assess what is needed in terms of technology transfer, project financing and scale-up.
Roll-out and uptake. African countries will need efficient processes to fast-track market authorization of safe and effective COVID-19 vaccines. CONCVACT is working out how to harmonize decisions of country-level regulators with global processes (such as the WHO pre-qualification programme) and expedite roll-out across the continent. This is likely to include indemnification provisions for manufacturers and a pharmacovigilance system to monitor adverse events and interface with national regulatory agencies.
Once a vaccine is approved, it needs to be delivered. Existing immunization systems in Africa are set up to vaccinate children, or to provide highly targeted ‘ring vaccination’ to families, neighbours and co-workers of people infected with diseases such as Ebola. To reach 60% of Africans, existing systems must be transformed to serve large numbers of adults and prioritize vulnerable populations, all without neglecting childhood vaccinations.
How poorer countries are scrambling to prevent a coronavirus disaster
In partnership with others, Africa CDC has set up the Africa Medical Supply Platform, a system to coordinate procurement that can be adapted for vaccine distribution. Working with national leaders, we have deployed thousands of community health workers and emergency responders across the continent to manage the test, trace and treat strategy. They will support the vaccination efforts of the African Union’s member states, and train others to do so. Another task is to work with existing distribution systems to deliver vaccines efficiently. For instance, refrigerated bottles of Coca-Cola are available in even the remotest areas of Africa. Our health systems should learn from, and even partner with, such commercial systems. We also need innovative technology to track distribution.
All of these preparations will fall flat if people are not willing to receive the vaccine, including follow-up doses. Trust therefore needs to be built up, to offset high levels of public-health misinformation and anti-vaccine sentiments. How? By giving communities access, information, counselling and support. The WHO has a WhatsApp messaging system dedicated to countering untruths about COVID‑19. We intend to launch continent-wide campaigns working with social-media platforms and marketing agencies. Also essential is engagement with senior national officials and the recruitment of local opinion-formers — including politicians, journalists, celebrities and religious leaders — to share accurate information and encourage safe vaccination. An Africa CDC technical working group is developing guidance for partnering with stakeholders to make public-education campaigns more effective, including using behavioural messaging to counter the ‘infodemic’.
The road ahead will be hard. The Africa Task Force for Coronavirus has been meeting weekly since February. Seven working groups focus on aspects including laboratory diagnosis and viral subtyping, case management, surveillance, risk communication, infection prevention and control, supply-chain management and scientific standards. Africa is seizing this opportunity to build up its public-health preparedness and response infrastructure at every level — continental, regional, national and local.
We hope that the rest of the world will follow our lead and embrace cooperation and multilateralism to overcome this pandemic and future ones. Infectious agents span the globe in weeks: vaccinating people on one continent is essential to the health, wealth and well-being of those on the others. No region can be immune until a meaningful and equitable share of the world’s population is protected — by the tenets of good basic public health as well as a vaccine.

 Let Africa into the market for COVID-19 diagnostics
Let Africa into the market for COVID-19 diagnostics
 How poorer countries are scrambling to prevent a coronavirus disaster
How poorer countries are scrambling to prevent a coronavirus disaster
 The unequal scramble for coronavirus vaccines — by the numbers
The unequal scramble for coronavirus vaccines — by the numbers