- NEWS AND VIEWS
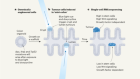
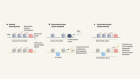
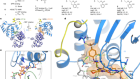
Seeds of cancer in normal skin
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 586, 504-506 (2020)
doi: https://doi.org/10.1038/d41586-020-02749-9
References
Tang, J. et al. Nature 586, 600–605 (2020).
Martincorena, I. et al. Science 348, 880–886 (2015).
McGrath, J. A. Eady, R. A. J. & Pope, F. M. in Rook’s Textbook of Dermatology (eds Burns, T., Breathnach, S., Cox, N. & Griffiths, C.) Ch. 3 (Blackwell, 2004).
Lodato, M. A. et al. Science 348, 880–886 (2015).
Welch, J. S. et al. Cell 150, 264–278 (2012).
Blokzijl, F. et al. Nature 538, 260–264 (2016).
Yoshida, K. et al. Nature 578, 266–272 (2020).
Jaiswal, S. et al. N. Eng. J. Med. 371, 2488–2498 (2014).
Kennedy, S. R., Zhang, Y. & Risques, R. A. Trends Cancer 5, 531–540 (2019).
Huang, F. W. et al. Science 339, 957–959 (2013).

 Read the paper: The genomic landscapes of individual melanocytes from human skin
Read the paper: The genomic landscapes of individual melanocytes from human skin
 Smoke signals in the DNA of normal lung cells
Smoke signals in the DNA of normal lung cells
 Mutations differ in normal and cancer cells of the oesophagus
Mutations differ in normal and cancer cells of the oesophagus