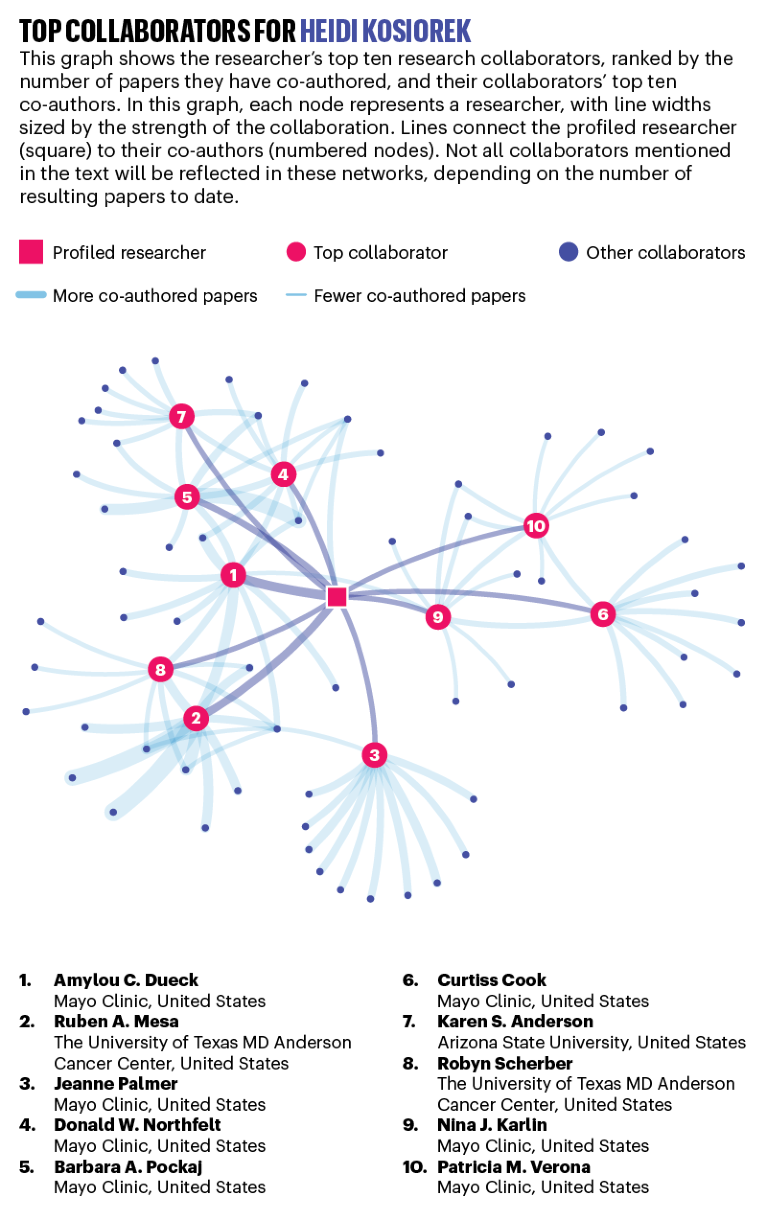
Biostatistician Heidi Kosiorek works at the crossroads of maths and medicine, sifting through data to weigh the best course of action based on cancer types and patient profiles. Credit: Melissa Valladares for Nature
“It’s impossible to do cancer research on your own if you want to do something that makes sense for the disease,” says lung cancer researcher, Niki Karachaliou, referring to the diverse teams of physicians, clinicians and other researchers whose different perspectives help create a shared understanding of this complex disease.
Karachaliou and two other researchers whose collaboration networks are shown here were selected for the strength of their publication count in Dimensions. They were drawn from an elite group of researchers in cancer, who were authors in Nature Index between 2015 and 2019 and whose first authorship on an article in Dimensions dates between 2010 and 2014. Authors from the United States dominate the group, as might be expected, given the US leads the field. Although the closest connections for two of the collaboration networks shown here are domestic, the third reflects activity between the United States and China, the top two countries for cancer research.
Among the top five countries for cancer research in the index, the United States and China are the most self-sufficient, with internationally collaborative articles on cancer comprising only 51.8 and 49.8%, respectively, of their total cancer articles. For the third-ranked country, the United Kingdom, 81.6% of cancer articles are internationally collaborative, and for Germany, fourth, 80.7% of cancer articles share authorship with researchers outside Germany.
HEIDI KOSIOREK: Number cruncher
Annual average publications count: 30.2
Predicting how various treatments could affect an individual cancer patient requires more than a medical understanding of the genetics and molecular mechanisms underlying the disease. It involves sifting through reams of data to identify, by weight of numbers, the tumour and patient characteristics that could influence success or failure. It’s about separating the trends from the flukes, the biomarkers from the outliers.
This first occurred to Heidi Kosiorek in the mid-1990s, during an internship working as a research assistant in the emergency department of an Ohio hospital. “I think it’s even more true today” at a time when personalized and precision medicine have become buzzwords, says Kosiorek, now a biostatistician at the Mayo Clinic’s Scottsdale, Arizona, campus.
Kosiorek had planned to go to medical school, but with her eyes opened to the intersection between mathematics, which had long been her strength, and medicine, she pursued biostatistics instead. That decision kicked off a prolific career. Since 2015, Kosiorek has authored or co-authored an average of 30 publications per year. For more than a decade, she worked at the University Hospitals Case Medical Center in Cleveland on studies of ovarian, endometrial and cervical cancers. Since joining the Mayo Clinic in late 2014, she’s developed expertise in breast cancer, and often teams up with Mayo Clinic colleagues, Barbara Pockaj, a surgeon, and Donald Northfelt, a medical oncologist, to investigate topics such as tumour genetics and detecting recurrence.
The Mayo Clinic receives nearly US$120 million in annual grant funding for cancer research, keeping biostatisticians at its three major campuses busy. Choosing which projects to work on “is a challenge, for sure”, says Kosiorek, “because I want to do it all”.
As an assistant supervisor, Kosiorek helps assign roughly 20 statisticians and statistical programmers to projects and oversees their work. Her mentorship of many junior scientists partly explains her impressive publication rate. “You end up being a part of more projects because of that,” she says.
Kosiorek’s collaborations extend beyond the Mayo Clinic. She is the lead biostatistician for the Myeloproliferative Neoplasms Research Consortium, a US National Cancer Institute-funded initiative to improve treatment for a rare chronic blood cancer that sometimes develops into leukaemia. She works closely with researchers from several institutions across the United States and Canada that are part of the consortium.
A crucial part of individualized medicine is taking into account the patient’s preferences regarding outcomes and quality-of-life impacts. With that in mind, Kosiorek and Pockaj are running a study on follow-up surgeries after breast reconstruction. They want to categorize the kinds of procedures that are needed for cosmetic considerations, for example, and how often, so that breast-cancer patients can make more informed decisions when considering reconstruction after a mastectomy.
“What drives me is helping physicians find what’s best for their patients through the data,” Kosiorek says. “There are many days where it doesn’t really feel like work.” — by Sarah DeWeerdt
Source: Nature Index/Dimensions, an interlinked research information system provided by Digital Science (https://www.dimensions.ai)
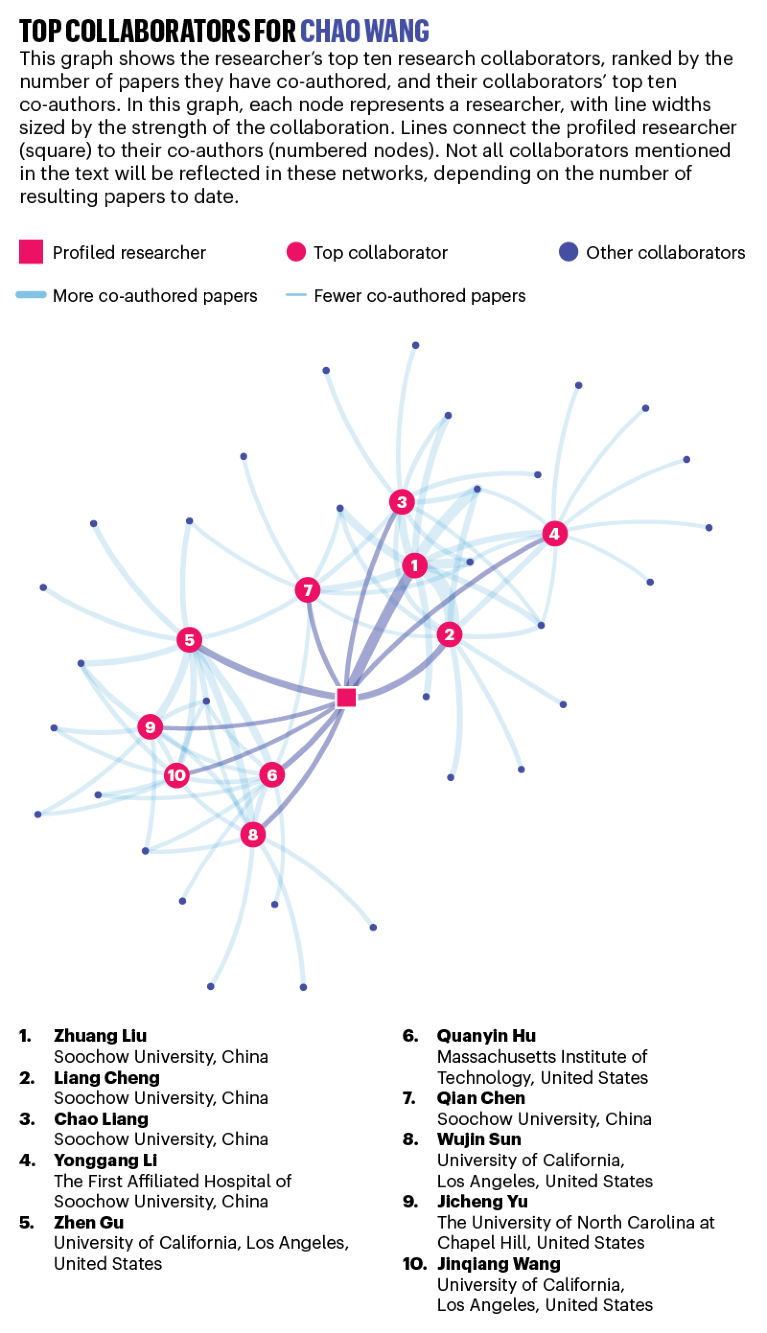
CHAO WANG: Biomedical naturalist
Annual average publications count: 13.4

Chao Wang.Credit: Chao Wang Lab/Soochow University
Immunotherapy, which harnesses the immune system to attack tumour cells, is one of the hottest fields in cancer research. One approach involves attaching immune-boosting drugs to nanoparticles made of gold or iron oxide and injecting them into the patient.
In his pursuit of new cancer-fighting drugs, biomedical engineer Chao Wang eschews such synthetic materials and looks instead to more organic drug carriers — the body’s own cells. “Nature is the best engineer,” he says.
In addition to being expensive to produce, metallic nanoparticles could potentially be toxic over the long term, says Wang. He’s investigating the humble red blood cell, the most abundant cell type in the human body, as a safer alternative. “Red blood cells may be the ideal carrier,” he says.
In 2019, Wang and his team described how immune-stimulating molecules called antigens were administered to mice using their own red blood cells. The cells were extracted, modified with antigens, and then re-injected, where they were taken up by the spleen. As reported in Science Advances1, the treatment spurred the immune systems of the mice, which helped to slow the tumour growth and increase survival rates. This was the first major result from Wang’s lab since its launch in 2018 at Soochow University in Suzhou, China, roughly 100 kilometres west of Shanghai.
In principle, different types of cells could be used to target the immune system in different parts of the body. For instance, fresh red blood cells, which are responsible for circulating oxygen in the body, could be used as transport for drugs that target the lungs. “We can do a lot of fancy things with these simple cells,” says Wang.
Before he moved to Suzhou, Wang was a postdoctoral fellow in the United States, where he worked with his adviser, Zhen Gu, then at the University of North Carolina at Chapel Hill, to assess the potential of platelets (tiny blood cells) to deliver a class of drugs called immune checkpoint inhibitors.
Checkpoint inhibitors block the mechanisms that usually keep the immune system in check, allowing it to be unleashed on cancer cells. But this can have serious side effects if it also attacks healthy cells. Because platelets naturally migrate to sites of inflammation, they can carry checkpoint inhibitors to a more targeted site. Surgical wounds, for example, where a tumour has been removed are a good place to tackle any residual cancer cells.
According to their 2017 Nature Biomedical Engineering paper2, Wang and Gu’s platelet therapy technique reduced cancer recurrence in mice, allowing 75% to survive after 60 days. No mice in the control group survived.
In North Carolina, Wang says he learnt the importance of working closely with clinicians and doctors, including bringing them into group meetings. It’s a philosophy he took back to Soochow, where he works with professors, doctors and students from the university’s medical school. “You cannot just do it in your lab by yourself,” he says. “You need contact with the clinicians and the doctors to know which problems you want to address in real life.” — by Mark Zastrow
Source: Nature Index/Dimensions, an interlinked research information system provided by Digital Science (https://www.dimensions.ai)
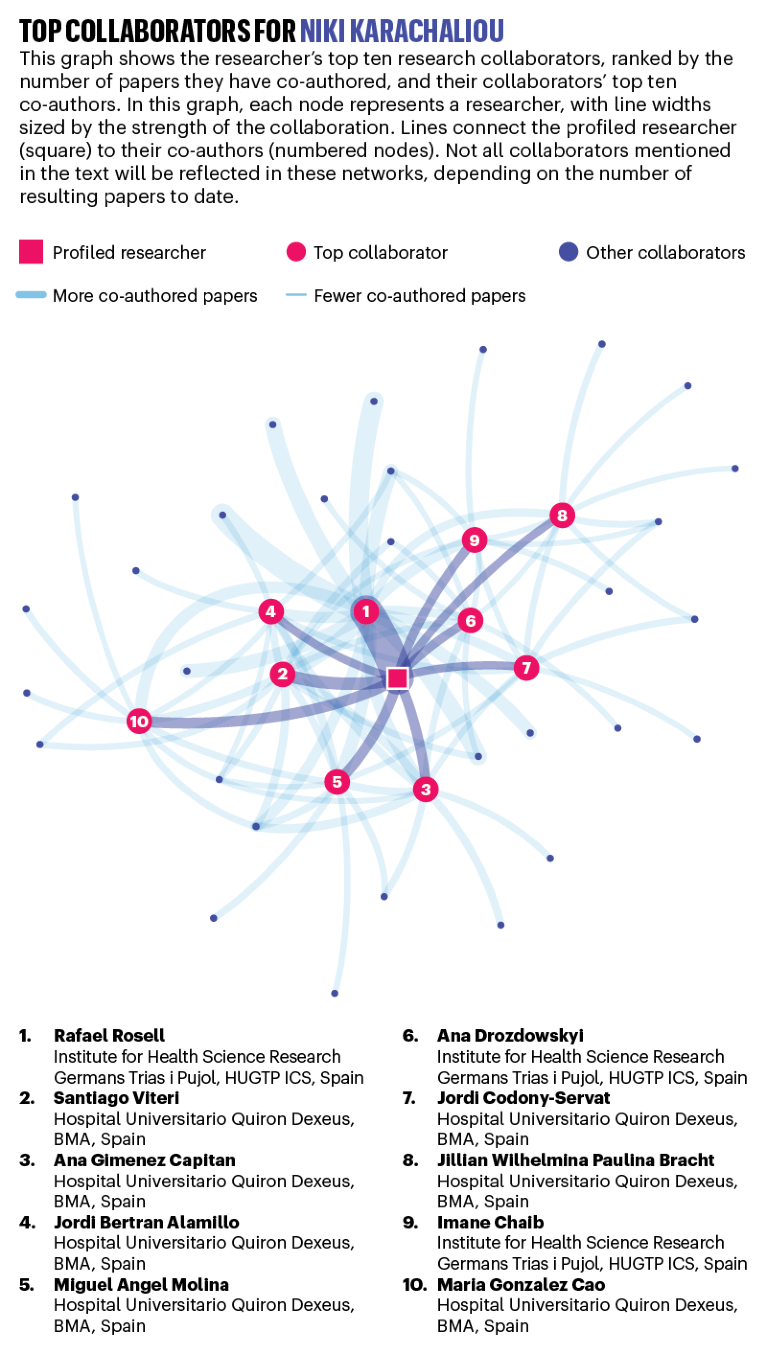
NIKI KARACHALIOU: Target hunter
Annual average publications count: 25.4

Niki Karachaliou searches for the mechanism that allows tumours to resist treatment. Credit: Linda Pudelko
Lung cancer kills more people every year than any other type of cancer worldwide, and smoking is its leading cause. But Niki Karachaliou’s research focuses on EGFR-positive lung cancer, which is more common among non-smokers than smokers, and caused by a mutation in the EGFR gene, which triggers rapid growth and division.
Although EGFR-inhibiting drugs such as gefitinib (sold as Iressa) and erlotinib (known as Tarceva) block the effects of this particular mutation, new treatment-resistant mutations tend to emerge within a year in almost all patients, says Karachaliou. This allows disease progression, leaving doctors with little course of action.
“There are still too many failures, with few patients responding well to targeted therapies,” says Karachaliou, who moved from Spain to become medical director of the GCD (Global Clinical Development) Oncology division at Merck, in Darmstadt, Germany, in 2019. “It’s devastating telling patients that we can only keep them disease-free for a few months.”
In 2019, Karachaliou’s team identified two potential drug targets in lung-cancer patients who have been treated unsuccessfully with EGFR-inhibitors3. By analysing tumour samples, they found that the disease progressed 6–12 months earlier in patients with high levels of mutated enzymes called ILK and SHP2. The findings are being used to develop treatments that complement EGFR therapies.
Using liquid biopsies, Karachaliou has also uncovered new classes of mutations in the BRAF gene, which produces a protein involved in cellular signalling. The liquid biopsy is a new test that captures changing tumour signatures in the blood, providing cancer researchers with a “more complete picture of what is happening at the time of progression”, says Karachaliou.
Collaboration has driven Karachaliou’s career since she graduated as a medical student from the University of Athens in Greece. She has co-authored more than 200 papers, many of which were with her collaborator and mentor, Rafael Rosell, who leads the Dr Rosell Oncology Institute in Barcelona, Spain. Karachaliou began working with Rosell at Barcelona’s Quirón Dexeus University Hospital in 2012, while she was completing her PhD.
Between patient visits and lab work, Karachaliou worked with a diverse team of clinicians, physicians and other researchers. “It was very interactive, which is important in oncology,” says Karachaliou. “It’s impossible to do cancer research on your own if you want to do something that makes sense for the disease.” — by Gemma Conroy
Source: Nature Index/Dimensions, an interlinked research information system provided by Digital Science (https://www.dimensions.ai)


 A global drive to eliminate cervical cancer
A global drive to eliminate cervical cancer
 Worth the cost? A closer look at the da Vinci robot’s impact on prostate cancer surgery
Worth the cost? A closer look at the da Vinci robot’s impact on prostate cancer surgery
 Game-changing class of immunotherapy drugs lengthens melanoma survival rates
Game-changing class of immunotherapy drugs lengthens melanoma survival rates