- NEWS AND VIEWS
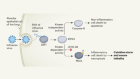
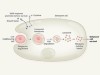
Ghostly metabolic messages from dying cells
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 580, 36-37 (2020)
doi: https://doi.org/10.1038/d41586-020-00641-0
References
Medina, C. B. et al. Nature 580, 130–135 (2020).
Green, D. R. Cell Death: Apoptosis and Other Means to an End 2nd edn (Cold Spring Harb. Lab. Press, 2018).
Lüthi, A. U. & Martin, S. J. Cell Death Differ. 14, 641–650 (2007).
Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. Br. J. Cancer 26, 239–257 (1972).
Henson, P. M. Annu. Rev. Cell Dev. Biol. 33, 127–144 (2017).
Garg, A. D. & Agostinis, P. Immunol. Rev. 280, 126–148 (2017).
Bosurgi, L., Hughes, L. D., Rothlin, C. V. & Ghosh, S. Immunol. Rev. 280, 8–25 (2017).
Chekeni, F. B. et al. Nature 467, 863–867 (2010).
Haskó, G., Sitkovsky, M. V. & Szabó, C. Trends Pharmacol. Sci. 25, 152–157 (2004).
Haskó, G. et al. J. Immunol. 164, 1013–1019 (2000).
Orozco, S. L. et al. Cell Rep. 28, 2275–2287 (2019).
Competing Interests
The author is a consultant for Inzen Pharmaceuticals and is on the scientific advisory board of Ventus Therapeutics.

 Read the paper: Metabolites released from apoptotic cells act as tissue messengers
Read the paper: Metabolites released from apoptotic cells act as tissue messengers
 A powerful cell-protection system prevents cell death by ferroptosis
A powerful cell-protection system prevents cell death by ferroptosis
 Senescent cells feed on their neighbours
Senescent cells feed on their neighbours