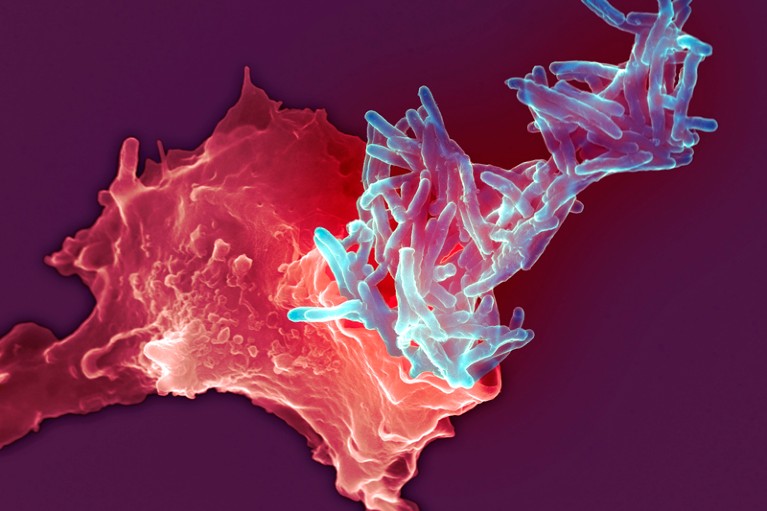
A white blood cell (red) engulfing the Mycobacterium bovis bacteria (blue) used in the BCG vaccination for tuberculosis.Credit: SPL
Tuberculosis is a leading cause of death worldwide and the biggest killer among people with HIV. Despite widespread efforts to boost vaccination and treatment, each year around 10 million people become ill from the disease, and in 2018 some 1.5 million people died, according to the World Health Organization (WHO). Astonishingly, the only licensed tuberculosis vaccine — BCG (Bacillus Calmette–Guérin), made from a weakened strain of a tuberculosis bacterium — has been in use for almost a century. Although BCG ably protects infants from systemic infection, it does less well against the lung disease that tuberculosis causes in adolescents and adults.
Newer anti-tuberculosis medicines have been developed, and more drugs and vaccines are in clinical trials. Nonetheless, the two most powerful drugs currently in use are not effective against multidrug-resistant strains of the disease-causing bacterium, Mycobacterium tuberculosis.
A new study in non-human primates (P. A. Darrah et al. Nature 577, 95–102; 2020) could breathe new life into the BCG vaccine. The key advance it reports is that delivery of the vaccine intravenously — directly into the bloodstream — rather than into skin or muscle, boosted protection against tuberculosis, particularly in the lungs. The translation of those findings into clinical practice is still some way off, but the pay-off could be huge.
In the study, researchers in the United States compared the results when BCG was delivered in three different ways to separate groups of macaque monkeys (Macaca mulatta): intravenously; by injection into the skin; or through an aerosol spray. The team found that intravenous administration yielded more T cells — a type of immune cell — that responded to M. tuberculosis than did either of the other routes. Moreover, these T cells were found throughout the animals’ lungs.
Six months later, some of the macaques were exposed to M. tuberculosis. Of those who received the vaccine intravenously, nine out of ten animals were highly protected against disease and six showed no detectable signs of infection. By contrast, most of the macaques vaccinated by other routes exhibited hallmarks of infection.
The results were a surprise: it was thought that intravenous delivery of the vaccine might limit the severity of disease, but not prevent infection altogether. Instead, intravenous BCG delivery seems to trigger the establishment of a population of T cells in the lungs that can rapidly neutralize M. tuberculosis.
If these results hold up in subsequent studies, this would represent significant promise for tuberculosis control. A cheap and already widely used vaccine could confer greater resistance to disease with only a relatively small change to the way in which it is administered. But there are a number of hurdles to overcome.
The first is safety. As with most vaccines, BCG contains live bacteria that have been weakened, making them unable to infect people with robust immune systems. But the risks of injecting a live bacterium directly into the bloodstream — as opposed to injecting it into the skin — need to be better understood. If, for some reason, a vaccine became contaminated by other bacteria or contained M. tuberculosis capable of causing disease, an infection could quickly become life-threatening. Researchers must complete careful and exhaustive safety tests before carrying the technique over into clinical trials in humans.
Further obstacles
If the new approach is found to be safe, testing intravenous administration of the vaccine and then delivering it in people is likely to present further obstacles. It is more difficult to administer a vaccine intravenously than to give a quick jab into skin or muscle — and doing so will require extra funds to train medical personnel in countries where underfunded public-health systems already struggle to provide adequate primary health care.
This lack of funding, and the fact that tuberculosis mostly affects people in poorer countries, is one reason why drug companies have not developed new treatments. However, newer players — particularly not-for-profit research groups — are changing drug discovery. One leading vaccine candidate, M72/AS01E, developed through a collaboration between a non-profit organization and a pharmaceutical company, protected adults with latent M. tuberculosis infection from going on to develop active tuberculosis for at least three years (D. R. Tait et. al. N. Engl. J. Med. 381, 2429–2439; 2019). And last summer, the US Food and Drug Administration approved a drug — also developed through not-for-profit research — as part of a combination to treat an extreme form of multidrug-resistant tuberculosis. In all, the WHO says that there are 23 drugs, several combination therapies and 14 vaccine candidates in clinical trials.
Intravenous vaccines are being developed in other areas, too. One malaria vaccine called PfSPZ is due to be tested in a large clinical trial in 2,100 people this year in Bioko, Equatorial Guinea.
Existing tuberculosis treatments saved between 53 million and 64 million lives between 2000 and 2018. But it is unacceptable that so many people still fall ill with the disease. Under the United Nations Sustainable Development Goals, tuberculosis deaths need to be reduced by 90% by 2030, and BCG vaccination will be a crucial part of this effort. Universal vaccination in infants has been recommended in more than 150 countries — including many in Africa and southeast Asia, which have been particularly hard hit by the disease. A potential means of boosting a vaccine’s power warrants the world’s attention.

 Tuberculosis vaccine finds an improved route
Tuberculosis vaccine finds an improved route
 Read the paper: Prevention of tuberculosis in macaques after intravenous BCG immunization
Read the paper: Prevention of tuberculosis in macaques after intravenous BCG immunization
 Treatment for extreme drug resistant tuberculosis wins US government approval
Treatment for extreme drug resistant tuberculosis wins US government approval