- NEWS AND VIEWS
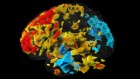
Cell-tracking pipeline reveals how motor circuits are built
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 576, 46-47 (2019)
doi: https://doi.org/10.1038/d41586-019-03492-6
References
Wan, Y. et al. Cell 179, 355–372 (2019).
Hanson, M. G. & Landmesser, L. T. J. Neurosci. 23, 587–600 (2003).
Gosgnach, S. et al. J. Neurosci. 37, 10835–10841 (2018).
D’Elia, K. P. & Dasen, J. S. Neural Dev. 13, 10 (2018).
Baek, M., Pivetta, C., Liu, J.-P., Arber, S. & Dasen, J. S. Cell Rep. 21, 867–877 (2017).
Song, J., Ampatzis, K., Björnfors, E. R. & El Manira, A. Nature 529, 399–402 (2016).
Warp, E. et al. Curr. Biol. 22, 93–102 (2012).
Gao, P., Sultan, K. T., Zhang, X.-J. & Shi, S. H. Development 140, 2645–2655 (2013).
Mayer, C. et al. Neuron 87, 989–998 (2015).
Holguera, I. & Desplan, C. Science 362, 176–180 (2018).

 Developing neurons are innately inclined to learn on the job
Developing neurons are innately inclined to learn on the job
 A deep dive into the development of sea squirts
A deep dive into the development of sea squirts