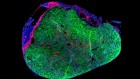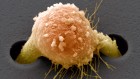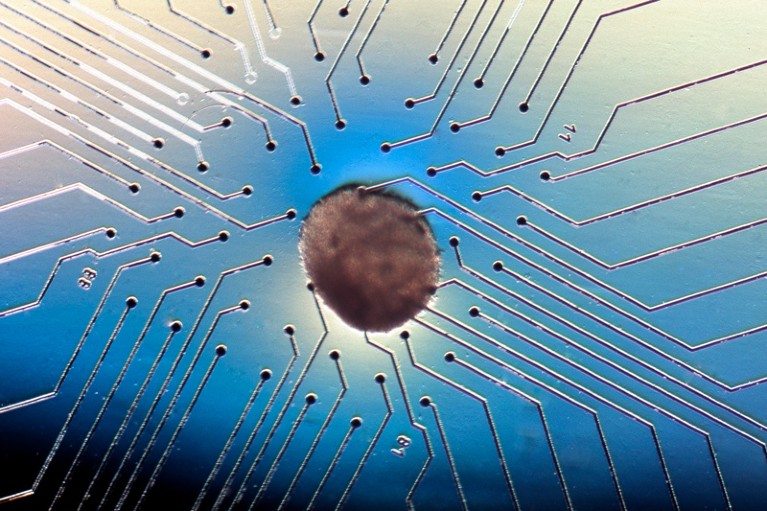
These heart cells on an electrocardiogram chip have been derived from human embryonic stem cells.Credit: Volker Sterger/SPL
In the global race to create companies offering stem-cell therapies, one country is looking to stand out from its competitors — Japan.
It is five years since Japan passed laws regulating stem-cell clinics; in that time, some 3,700 treatments have received the green light. From Hokkaido to the islands of Okinawa, companies in Japan can extract stem cells from skin biopsies and use them in injections for complex conditions such as heart disease.
But the vast majority of these therapies have not passed a randomized, controlled, double-blind clinical trial, the global standard to prove that interventions are safe and effective, and the foundation for most medical regulation. Instead, Japan’s 2014 Act on the Safety of Regenerative Medicine and a second law, the 2014 Pharmaceutical and Medical Device Act, provide a fast track to market approval.
The potent effects of Japan’s stem-cell policies
These laws were passed following the award of the 2012 Nobel Prize in Physiology or Medicine to Kyoto University stem-cell biologist Shinya Yamanaka. The government of Prime Minister Shinzo Abe decided to establish one of the world’s more liberal regulatory environments for regenerative medicine.
But it isn’t only Japanese companies that are in a rush to commercialize stem-cell treatments. The country is becoming a magnet for scientists and entrepreneurs from around the world who are seeking a rapid route to commercializing products and therapies .
Japan’s attractiveness to regenerative-medicine entrepreneurs is prompting other countries to look closely at its regulatory changes. There is undoubtedly a competition under way, and unless something is done, it risks becoming a race to the bottom.
Supporters of Japan’s laws justify the fast-track approvals system by arguing that more conventional regulations would drive clinics underground, and regulators would constantly have to work to catch up — as is the case for the US Food and Drug Administration. Japan’s solution, they argue, means that companies are compelled to operate in the public eye, which is itself a form of transparency, because clinics are visible and not hidden.
Moreover, the law requires stem cells to be processed in high-quality, certified cell-processing centres, and treatments to pass through an independent ethical-review board — there are 100 of these. An official in Japan’s Ministry of Health, Labour and Welfare told Nature that double-blind clinical trials are expensive, and that there are ethical issues involved in giving placebos to people with illnesses.
It is possible that some of these justifications have a degree of merit, but there’s still no denying that the majority of commercially available stem-cell therapies have not been tested in more rigorous phased clinical trials.
Japan should put the brakes on stem-cell sales
That leads to a second concern. As with all medical therapies, people regard government approval for stem-cell clinics as reassurance that treatments they offer are both safe and viable. Unless people have read the text of the law, they will not know that stem-cell products and therapies have a low barrier to regulatory approval. One doctor told Nature that, from a patient’s perspective, an approval is an approval, and “everything else is just details”.
Japan’s dilemma is a global one. Every government can see a pot of gold at the end of the stem-cell rainbow, but countries know that these riches cannot come at the expense of increased risks to patient safety.
Regulators in the United States, who have also struggled with these issues, are adhering to the international regulatory consensus for medical therapies, and seem to be getting the upper hand in their battles against treatments that have not been rigorously tested.
Japan’s government must rethink its approach, and those looking to the nation’s present laws as a regulatory role model must also think again.
The world needs the pioneering research that Japan and other countries conduct in stem-cell biology — and it needs promising therapies for chronic disease. But getting from one to the other takes time, and rigorous safeguards should not be circumvented. Policymakers, regulators, researchers and entrepreneurs taking short cuts are potentially putting people’s health at risk.

 The potent effects of Japan’s stem-cell policies
The potent effects of Japan’s stem-cell policies
 Japan should put the brakes on stem-cell sales
Japan should put the brakes on stem-cell sales
 Woman is first to receive cornea made from ‘reprogrammed’ stem cells
Woman is first to receive cornea made from ‘reprogrammed’ stem cells