- NEWS AND VIEWS
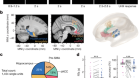
Lymphatic vessels at the base of the mouse brain provide direct drainage to the periphery
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 572, 34-35 (2019)
doi: https://doi.org/10.1038/d41586-019-02166-7
References
Louveau, A. et al. J. Clin. Invest. 127, 3210–3219 (2017).
Bower, N. I. & Hogan, B. M. J. Mol. Med. 96, 383–390 (2018).
Aspelund, A. et al. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Nature 523, 337–341 (2015).
Da Mesquita, S. et al. Nature 560, 185–191 (2018).
Louveau, A. et al. Nature Neurosci. 21, 1380–1391 (2018).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Nature Commun. 8, 1434 (2017).
Ahn, J. H. et al. Nature 572, 62–66 (2019).
Antila, S. et al. J. Exp. Med. 214, 3645–3667 (2017).
Maisel, K., Sasso, M. S., Potin, L. & Swartz, M. A. Adv. Drug Deliv. Rev. 15, 43–59 (2017).
Baluk, P. et al. J. Exp. Med. 204, 2349–2362 (2007).

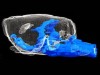 Read the paper: Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid
Read the paper: Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid
 A lymphatic waste-disposal system implicated in Alzheimer’s disease
A lymphatic waste-disposal system implicated in Alzheimer’s disease
 Leukaemia follows a blood-vessel track to enter the nervous system
Leukaemia follows a blood-vessel track to enter the nervous system