- NEWS AND VIEWS
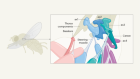
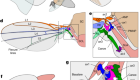
Cell communication in the blink of an eye
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 571, 484-485 (2019)
doi: https://doi.org/10.1038/d41586-019-02069-7
References
Mathijssen, A. J. T. M., Culver, J., Bhamla, M. S. & Prakash, M. Nature 571, 560–564 (2019).
Buonanno, F., Guella, G., Strim, C. & Ortenzi, C. Hydrobiologia 684, 97–107 (2012).
Haeckel, E. Das protistenreich. Eine populäre uebersicht über das formengebiet der niedersten lebewesen (Günther, 1878); https://doi.org/10.5962/bhl.title.58542
Jones, A. R., Jahn, T. L. & Fonseca, J. R. J. Cell. Physiol. 68, 127–133 (1966).
Hawkes, R. B. & Holberton, D. V. J. Cell. Physiol. 84, 225–235 (1974).
Lehman, W. J. & Rebhun, L. I. Protoplasma 72, 153–178 (1971).
Ishida, H. & Shigenaka, Y. Cytoskeleton 9, 278–282 (1988).
Yagiu, R. & Shigenaka, Y. J. Protozool. 10, 364–369 (1963).
Winkler, R. G. Eur. Phys. J. Spec. Top. 225, 2079–2097 (2016).
Cortez, R., Fauci, L. & Medovikov, A. Phys. Fluids 17, 031504 (2005).
Phillips, R., Kondev, J., Theriot, J. & Garcia, H. G. Physical Biology of the Cell (Garland, 2012).
Ohmura, T. et al. Proc. Natl Acad. Sci. USA 115, 3231–3236 (2018).
Xia, W. & Thorpe, M. F. Phys. Rev. A 38, 2650–2656 (1988).
Takeuchi, K. A., Kuroda, M., Chaté, H. & Sano, M. Phys. Rev. Lett. 99, 234503 (2007).
Balanis, C. A. Proc. IEEE 80, 7–23 (1992).
Buonanno, F. Biologia 66, 648–653 (2011).

 Read the paper: Collective intercellular communication through ultra-fast hydrodynamic trigger waves
Read the paper: Collective intercellular communication through ultra-fast hydrodynamic trigger waves
 Evolutionary race as predators hunt prey
Evolutionary race as predators hunt prey
 How fish feel the flow
How fish feel the flow