- NEWS AND VIEWS
Nerve cells from the brain invade prostate tumours
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 569, 637-638 (2019)
doi: https://doi.org/10.1038/d41586-019-01461-7
References
Ayala, G. E. et al. Clin. Cancer Res. 14, 7593–7603 (2008).
Magnon, C. et al. Science 341, 1236361 (2013).
Zhao, C. M. et al. Sci. Transl. Med. 6, 250ra115 (2014).
Stopczynski, R. E. et al. Cancer Res. 74, 1718–1727 (2014).
Peterson, S. C. et al. Cell Stem Cell 16, 400–412 (2015).
Hayakawa, Y. et al. Cancer Cell 31, 21–34 (2017).
Dobrenis, K., Gauthier, L. R., Barroca, V. & Magnon, C. Int. J. Cancer 136, 982–988 (2015).
Mauffrey, P. et al. Nature 569, 672–678 (2019).
Gonçalves, J. T., Schafer, S. T. & Gage, F. H. Cell 167, 897–914 (2016).
Kempermann, G. et al. Cell Stem Cell 23, 25–30 (2018).
Moreno-Jiménez, E. P. et al. Nature Med. 25, 554–560 (2019).
Sorrells, S. F. et al. Nature 555, 377–381 (2018).
Eriksson, P. S. et al. Nature Med. 4, 1313–1317 (1998).
Spalding, K. L. et al. Cell 153, 1219–1227 (2013).

 Read the paper: Progenitors from the central nervous system drive neurogenesis in cancer
Read the paper: Progenitors from the central nervous system drive neurogenesis in cancer
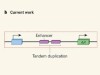 Sequence of events in prostate cancer
Sequence of events in prostate cancer
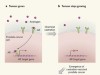 Resistance to prostate-cancer treatment is driven by immune cells
Resistance to prostate-cancer treatment is driven by immune cells