- NEWS AND VIEWS
Conformation of the native HIV-1 envelope protein raises questions for vaccine design
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 568, 321-322 (2019)
doi: https://doi.org/10.1038/d41586-019-01085-x
References
Lu, M. et al. Nature 568, 415–419 (2019).
Pancera, M., Changela, A. & Kwong, P. D. Curr. Opin. HIV AIDS. 12, 229–240 (2017).
Sanders, R. W. & Moore, J. P. Immunol. Rev. 275, 161–182 (2017).
Ward, A. B. & Wilson, I. A. Immunol. Rev. 275, 21–32 (2017).
Kadelka, C. et al. J. Exp. Med. 215, 1589–1608 (2018).
Munro, J. B. et al. Science 346, 759–763 (2014).
Sok, D. et al. Nature 548, 108–111 (2017).

 Read the paper: Associating HIV-1 envelope glycoprotein structures with states on virus observed by smFRET
Read the paper: Associating HIV-1 envelope glycoprotein structures with states on virus observed by smFRET
 Combination treatment prevents HIV re-emergence in monkeys
Combination treatment prevents HIV re-emergence in monkeys
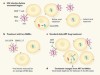 Antibodies pose a double threat to HIV
Antibodies pose a double threat to HIV