FUNDING
US budget request On 11 March, US President Donald Trump released his budget request for the 2020 fiscal year, which begins on 1 October 2019. The plan seeks to cut the National Institutes of Health budget to US$34.4 billion, roughly $5 billion below the current level, and funding for the Environmental Protection Agency to $6.1 billion, a $2.7 billion decrease. The White House wants to cut the National Science Foundation’s budget by $1 billion, to $7.1 billion. The proposal for NASA sets a goal of sending people to the Moon’s surface by 2028, and includes $363 million for a lunar lander that could carry cargo and astronauts. But it is unclear whether Congress will take Trump’s wishes into account as it formulates 2020 spending bills. The president proposed significant cuts to science funding for the 2018 and 2019 funding cycles, which lawmakers largely ignored.
FACILITIES
Nations sign giant-telescope treaty Nations involved in the Square Kilometre Array (SKA) project to build the world’s largest radio telescope have signed a treaty to establish a intergovernmental organization that will oversee the work and formally approve construction. The body, called the SKA Observatory, will be similar to organizations such as CERN, Europe’s particle-physics lab near Geneva, Switzerland. The powerful telescope, which will be built in phases, will eventually comprise thousands of radio dishes in Africa and up to one million antennas in Australia. Together, they will have a receiving area of 1 square kilometre and should be able to detect signals from the early Universe (pictured, prototype dishes in South Africa). The first phase will cost €674 million (US$760 million). Although 12 nations are partners in SKA, only seven, including Portugal and China, have signed the convention.

A radiodish of the MeerKAT telescope network, a test-bed for the Square Kilometre Array.Credit: South African Radio Astronomy Observ.
Collider delay Japan’s government has said that it is not ready to commit to hosting the world’s next major particle accelerator, the planned US$7-billion International Linear Collider (ILC), which has been more than a decade in the making. The ILC would be a straight, 20-kilometre collider that would study the Higgs boson. Only Japanese physicists have shown an interest in building the collider, but despite years of discussions, the government has yet to indicate any willingness to host, deterred in part by the cost. On 7 March, Japan’s science ministry told physicists overseeing the project that more discussion was needed, and called for talks about sharing costs with other countries. The delay will make it harder for European physicists to prioritize the project in their long-term strategy, which is currently being devised.
New LHC detector The Large Hadron Collider (LHC) will get a new way to hunt for exotic particles from 2021, after CERN, Europe’s particle-physics laboratory near Geneva, Switzerland, approved the construction of an experiment on 5 March. The Forward Search Experiment (FASER) will look for light, weakly interacting particles that, if produced in collisions, would fly through existing detectors but decay into known particles half a kilometre away. The diminutive, 20-centimetre-wide cylindrical detector will be installed in a disused tunnel at the LHC in 2020. FASER aims to detect hypothetical exotic particles including ‘dark photons’, which are associated with some proposed types of dark matter. The project is relatively cheap, at around 1 million Swiss francs (US$1 million) because it will use spare parts donated by two other LHC experiments.
HEALTH
Ketamine drug The US Food and Drug Administration (FDA) has approved esketamine — a form of the hallucinogenic party drug ketamine — for use as an antidepressant. It’s the first new type of drug to treat depression to reach the market in decades. Esketamine, a nasal spray developed by Johnson & Johnson of New Brunswick, New Jersey, will be sold under the name Spravato. The FDA’s 5 March announcement comes after an independent advisory council recommended the drug’s approval in early February, despite mixed results from Johnson & Johnson’s clinical trials and concerns about its potential for abuse. Esketamine is one of the two mirror-image molecules that make up ketamine, which also has antidepressant properties. Trials suggest that ketamine works in a matter of hours to alleviate the symptoms of depression, and has inspired several companies to make derivatives that imitate its effects.
PEOPLE
eLife editor Open-access journal eLife named geneticist Michael Eisen as its new editor-in-chief on 5 March. Eisen (pictured) takes over the prestigious title from founding editor-in-chief Randy Schekman, who stepped down in January. Eisen works at the University of California, Berkeley, and is a pioneer in the open-access publishing movement. In 2001, he co-founded the publisher Public Library of Science (PLOS), which was among the first in the world to charge authors a fee to publish their work in a way that meant anyone could read it for free. It now runs seven freely available titles, including the mega-journal PLoS ONE, and has published more than 215,000 articles. Eisen has published his own work only in open-access journals for nearly 20 years.

Biologist Michael Eisen has been appointed the editor of eLife.Credit: eLife
FDA head resigns Scott Gottlieb, the head of the US Food and Drug Administration (FDA), resigned on 5 March and will leave the agency in one month. A physician and venture capitalist who has served on the boards of several pharmaceutical companies, he became known at the FDA for his aggressive plan to crack down on the sale of flavoured e-cigarettes to curb vaping by underage children. On his watch, the Department of Health and Human Services, which oversees the FDA, initiated a review in September 2018 of all research involving fetal tissue and all acquisitions involving human fetal tissue. The department also cancelled a government contract with a company that supplied fetal tissue to FDA researchers. Gottlieb, who has led the FDA since May 2017, did not offer a reason for his departure in his resignation letter.
POLICY
Hybrid embryos Japan will allow scientists to grow human cells in an animal embryo and transfer it to an animal’s uterus, reversing a ban on the practice. The country’s science ministry announced the decision, which is effective immediately, on 1 March. The ultimate aim of the research is to use animals, such as pigs, to grow organs that can be transplanted into humans. The relaxed regulations would allow Hiromitsu Nakauchi, a stem-cell biologist at Stanford University in California and the University of Tokyo, to pursue experiments in Japan that he has planned for more than a decade, pending ethical approval. Nakauchi has created pig embryos that are genetically altered so that they cannot produce a pancreas, and he plans to insert induced pluripotent stem cells into the embryos. These cells are obtained by reprogramming human cells so that they revert to an embryonic-like state from which they can form other cell types. The hope is that the stem cells will develop into a pancreas composed mainly of human cells as the embryo develops.
UK committee The UK government is considering creating an research fund open to British and international scientists to fill a gap left by the loss of prestigious European Union funding after Brexit. Adrian Smith, director of the Alan Turing Institute in London, will lead a “major” project with the research community to look at establishing such a fund, UK science minister Chris Skidmore told a parliamentary science committee on 5 March. The move comes in response to concerns that, after Brexit, UK institutions may no longer be able to host prestigious European Research Council grants, which scientists around the world can apply for and take up at an EU institution, or fellowships called Marie Skłodowska Curie Actions that give researchers EU money to spend some time working in a lab in another country. It’s not yet clear whether UK-based researchers will have access to these schemes after Brexit.
TREND WATCH
Almost half of childhood cancer cases worldwide go undiagnosed, according to a model (Z. J. Ward et al. Lancet Oncol. http://doi.org/c3gr; 2019). Using data from the World Health Organization, researchers estimated that in 2015, 397,000 children under 15 developed cancer globally, and that 43% of those cases went undiagnosed. These figures are considerably higher than numbers from official cancer registries, say the scientists, suggesting that tens of thousands of children each year go without treatment, and potentially die from the disease without ever knowing they had it. Previous estimates had suggested that, worldwide, 200,000 children are diagnosed with cancer each year. But the true number is difficult to pin down because many nations do not record data. Even in countries that do have national registries, many cases might go undiagnosed because of poor access to health care. The model, which used registry, health-care and demographic data to make its estimates, also revealed vast disparities in diagnosis rates across the world. In western Europe and North America, for example, only 3% of cases are missed, whereas in south Asia and West Africa, estimates rise to 49% and 57%, respectively.
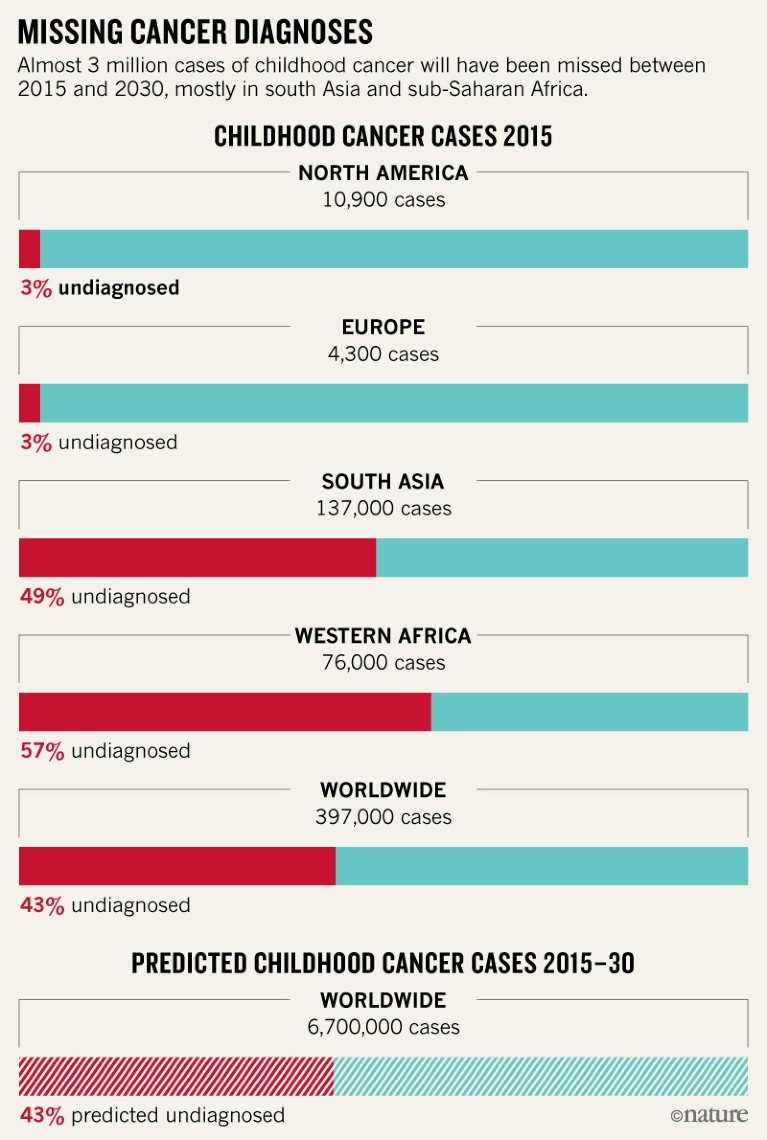
Source: Z. J. Ward et al. Lancet Oncol. http://doi.org/c3gr (2019)