- NEWS AND VIEWS
Pore-forming small molecules offer a promising way to tackle cystic fibrosis
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 567, 315-317 (2019)
doi: https://doi.org/10.1038/d41586-019-00781-y
References
Ratjen, F. et al. Nature Rev. Dis. Primers 1, 15010 (2015).
Muraglia, K. A. et al. Nature 567, 405–408 (2019).
Davies, J. C. et al. N. Engl. J. Med. 379, 1599–1611 (2018).
Keating, D. et al. N. Engl. J. Med. 379, 1612–1620 (2018).
Mall, M. A. & Galietta, L. J. V. J. Cystic Fibros. 14, 561–570 (2015).
Shen, B., Li, X., Wang, F., Yao, X. & Yang, D. PLoS ONE 7, e34694 (2012).
Li, H. et al. Nature Chem. 8, 24–32 (2016).
Stoltz, D. A., Meyerholz, D. K. & Welsh, M. J. N. Engl. J. Med. 372, 351–362 (2015).
Quinton, P. M. Am. J. Physiol. Cell Physiol. 299, C1222–C1233 (2010).
Anderson, T. M. et al. Nature Chem. Biol. 10, 400–406 (2014).
Cioffi, A. G., Hou, J., Grillo, A. S., Diaz, K. A. & Burke, M. D. J. Am. Chem. Soc. 137, 10096–10099 (2015).
Grillo, A. S. et al. Science 356, 608–616 (2017).
Diamond, J. M. Nature 300, 683–685 (1982).
Competing Interests
D.N.S. is the recipient of a Vertex Innovation Award funded by Vertex Pharmaceuticals. The awards are administered by Chameleon, a medical communications company. Vertex Innovation Awards are reviewed by a grant-awarding panel, which selects reviewers to assess the applications and makes a final decision based on the comments of peer reviewers. Members of the grant-awarding panel and peer reviewers are academics and/or clinicians independent of Vertex Pharmaceuticals.

 Read the paper: Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia
Read the paper: Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia
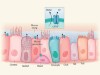 Profile of an unknown airway cell
Profile of an unknown airway cell
 Bacterial transmission tactics
Bacterial transmission tactics





