- NEWS AND VIEWS
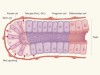
Gut immune cells have a role in food metabolism
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 566, 49-50 (2019)
doi: https://doi.org/10.1038/d41586-019-00235-5
References
He, S. et al. Nature 566, 115–119 (2019).
Ganusov, V. V. & De Boer, R. J. Trends Immunol. 28, 514–518 (2007).
McDonald, B. D., Jabri, B. & Bendelac, A. Nature Rev. Immunol. 18, 514–525 (2018).
Hoytema van Konijnenburg, D. P. et al. Cell 171, 783–794 (2017).
Rankin, L. C. & Artis, D. Cell 173, 554–567 (2018).
Drucker, D. J. Cell Metabolism 27, 740–756 (2018).
Yusta, B. et al. Diabetes 64, 2537–2549 (2015).

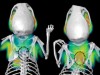 Read the paper: Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease
Read the paper: Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease
 Fat cells with a sweet tooth
Fat cells with a sweet tooth
 Debate over the identity of an intestinal niche-cell population settled
Debate over the identity of an intestinal niche-cell population settled