- COMMENT
Vaccine candidates for poor nations are going to waste
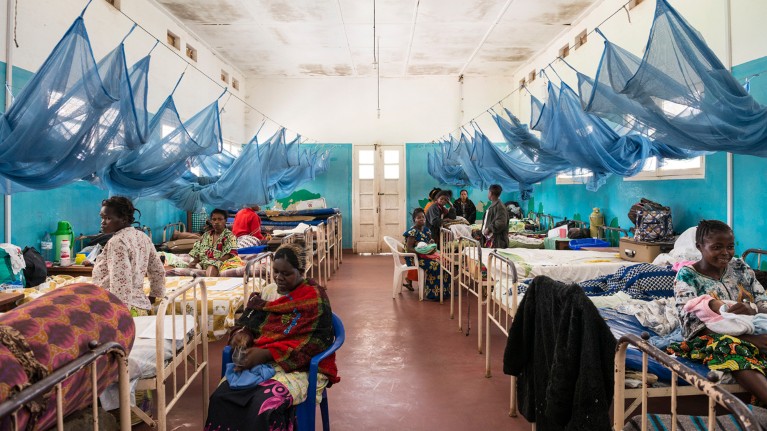
Women in a hospital ward with malaria bed nets in Bunia, the Democratic Republic of the Congo.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 564, 337-339 (2018)
doi: https://doi.org/10.1038/d41586-018-07758-3
References
Nussenzweig, R. S., Vanderberg, J., Most, H. & Orton, C. Nature 216, 160–162 (1967).
Ellis, J. et al. Nature 302, 536–538 (1983).
Cochrane, A. H., Santoro, F., Nussenzweig, V., Gwadz, R. W. & Nussenzweig, R. S. Proc. Natl Acad. Sci. USA 79, 5651–5655 (1982).
Stoute, J. A. et al. N. Engl. J. Med. 336, 86–91 (1997).
Alonso, P. L. et al. Lancet 364, 1411–1420 (2004).
RTS,S Clinical Trials Partnership. Lancet 386, 31–45 (2015).
Bloom, D. E., Fan, V. Y. & Sevilla, J. P. Sci. Transl. Med. 10, eaaj2345 (2018).
Trotter, C. L. et al. Lancet Infect. Dis. 17, 867–872 (2017).
Mustapha, M. M. & Harrison, L. H. Hum. Vaccin. Immunother. 14, 1107–1115 (2018).
Roberts, L. Science 320, 1710–1715 (2008).
Thompson, K. M. et al. Risk Anal. 26, 1571–1580 (2006).
Competing Interests
S.B. is a consultant for GSK, Takeda and Sutrovax.
D.E.B. has been in receipt of grants, travel grants, and/or personal fees from the Bill & Melinda Gates Foundation, the World Health Organization, Merck, Pfizer, GSK, Sanofi Pasteur, and Sanofi Pasteur-MSD (all related to his research on the value of vaccination).
M.D. is currently employed by a vaccine manufacturer, Biological E.
D.S. has acted as a paid consultant to vaccine manufacturers and was Chair of WHO’s Strategic Advisory Group of Experts 2006–10. He was Director of Immunisation at the Department of Health, London, until 2013.
R.R. is currently a full-time employee of GSK group of companies.

 Deploy vaccines to fight superbugs
Deploy vaccines to fight superbugs
 Nature special on vaccines
Nature special on vaccines