- NEWS AND VIEWS
Single-cell sequencing paints diverse pictures of the brain
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 563, 38-39 (2018)
doi: https://doi.org/10.1038/d41586-018-07027-3
References
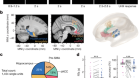
Tasic, B. et al. Nature 563, 72–78 (2018).
Economo, M. N. et al. Nature 563, 79–84 (2018).
Creutzfeldt, O. D. Naturwissenschaften 64, 507–517 (1977).
Nowakowski, T. J. et al. Science 358, 1318–1323 (2017).
Harris, K. D. & Shepherd, G. M. G. Nature Neurosci. 18, 170–181 (2015).
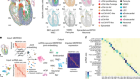
Zeisel, A. et al. Cell 174, 999–1014 (2018).
Saunders, A. et al. Cell 174, 1015–1030 (2018).
Hodge, R. D. et al. Preprint at bioRxiv https://doi.org/10.1101/384826 (2018).
Ecker, J. R. et al. Neuron 96, 542–557 (2017).

 Read the paper: Shared and distinct transcriptomic cell types across neocortical areas
Read the paper: Shared and distinct transcriptomic cell types across neocortical areas
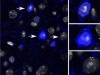 Read the paper: Distinct descending motor cortex pathways and their roles in movement
Read the paper: Distinct descending motor cortex pathways and their roles in movement
 Diversity reaches the stars
Diversity reaches the stars
 A mixed model of neuronal diversity
A mixed model of neuronal diversity