SPACE
Japan mission makes asteroid history Japan’s asteroid mission Hayabusa2 has become the first to land moving rovers on the surface of an asteroid. On 22 September, the Japan Aerospace Exploration Agency (JAXA) confirmed that the mission’s twin rovers, called MINERVA-II 1A and 1B, had landed safely on the space rock Ryugu, and that both were moving on the surface. The Hayabusa2 mothership temporarily dropped close to the surface (pictured) of the 1-kilometre-wide asteroid to deploy the probes last week. Mission controllers lost communication with the rovers in the hours after deployment, when the landers were on the asteroid’s far side, as seen from the orbiter. But the hexagonal rovers, which hop around slowly on the low-gravity surface, have now sent back their first blurry colour images. In October, the mothership is scheduled to touch down to collect a sample, which it will return to Earth. Scientists hope that studying the asteroid will help them to understand the origins of the Solar System’s planets.

Credit: JAXA
Gravity probe glitch A US–German gravity-mapping mission that launched in May has not gathered science data since mid-July because of a problem with a key instrument. The twin satellites of the Gravity Recovery and Climate Experiment Follow-On (GRACE-FO) mission measure minute differences in the distance between them to chart changes in Earth’s gravity. Doing so can reveal the distribution and flow of water and ice across the planet. But part of a microwave instrument aboard one of the satellites shut itself down on 19 July following signs of an electrical problem. Mission managers plan to switch to a back-up system later this month, said Frank Webb, the mission’s project scientist at NASA’s Jet Propulsion Laboratory in Pasadena, California, on 19 September.
POLICY
Clinical-trial fines The US Food and Drug Administration (FDA) is proposing heavy fines for pharmaceutical companies that fail to report clinical-trial results online. In a 20 September draft guidance statement, the FDA said that failure to register trials or submit results to the government database ClinicalTrials.gov could result in penalties of up to US$10,000 per day. The agency would send a notification letter to the company warning of an infraction. The company would then have 30 days to comply before facing fines. The public has 60 days to submit comments on the proposed rule. Studies have found that about 58% of industry-funded trials and 61% of trials funded by the US National Institutes of Health in the United States did not report their results five years after completion.
US biodefence The US government has revised its plans for responding to biological threats. On 18 September, the White House released the first US biodefence strategy that spans multiple government agencies. In another first, the plan covers not only deliberate bioterror threats, but also naturally occurring outbreaks and infectious diseases that escape a lab accidentally. The White House has also created a steering committee to coordinate operations in the event of a biological attack or a major disease outbreak; it will be led by the Department of Health and Human Services. It will undertake an immediate review of agencies’ biodefence strategies and capabilities in order to identify any gaps.
RESEARCH
Agriculture data A coalition including the Food and Agriculture Organization of the United Nations (FAO), the Bill and Melinda Gates Foundation and national governments has launched a US$500-million effort to help developing countries gather data on farmers and promote rural development. The initiative, announced on 24 September, will scale up surveys developed separately by the FAO and the World Bank that gather information on agriculture efforts around the world. The programme aims to fill a yawning information gap about what hundreds of millions of farmers living in poverty are doing across 50 countries in Africa, Asia and Latin America.
PEOPLE
Misconduct case Food psychologist Brian Wansink (pictured) has submitted his resignation to Cornell University in Ithaca, New York, and will retire at the end of the academic year, according to a 20 September statement from the university. The move comes as Cornell announced the findings of an internal investigation that concluded that Wansink had committed academic misconduct. Tha university’s year-long investigation found that the scientist had misreported research data, used “problematic” statistical techniques and failed to document his results properly. Cornell’s statement added that Wansink had been removed from teaching and research responsibilities, and would spend the remainder of the academic year cooperating with its ongoing review of his work. Wansink, who is known for his research on how external factors such as packaging influence what people eat, said that “there was no fraud, no intentional misreporting, no plagiarism, or no misappropriation” in an e-mailed statement to Nature.

Credit: Lefty Shivambu/Gallo Images/Getty
Scientist cleared An Indian space scientist, Nambi Narayanan, has been granted compensation after being falsely accused of trading secrets with a foreign country. On 14 September, India’s Supreme Court condemned the original police investigation against Narayanan and awarded 5 million rupees (US$68,600) to the former Indian Space Research Organisation scientist. In 1994, he was arrested on charges that he sold secret information on cryogenic rocket engines to Pakistan. Although India’s Central Bureau of Investigation and a local court cleared Narayanan of the allegations in 1996, the case was reopened by local authorities, which led to a protracted legal battle between various government agencies. The Supreme Court has now established a committee to investigate the police officers who arrested Narayanan.
FUNDING
Funds for children The US Department of Health and Human Services (HHS) plans to take money from research programmes to help house immigrant children whose parents were detained after entering the United States illegally. The HHS, which includes the US National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC), sent a letter to members of a Senate spending panel that oversees the agency, saying that it plans to transfer US$87.3 million from the NIH and $16.7 million from the CDC for the housing programme. The costs of housing these children have ballooned since April, when President Donald Trump instituted a policy that has led to the separation of many families, as part of a crackdown on illegal immigration. The letter was first reported on 19 September by Yahoo News.
HEALTH
Tuberculosis trends The incidence of tuberculosis (TB) is falling globally by about 2% per year, the World Health Organization said in a report on 18 September. Between 1.5 million and 1.7 million people are estimated to have died from TB in 2017, and 9 million to 11 million people developed the disease. India has the largest TB burden of any country, accounting for more than one-quarter of the world’s cases. Nearly one-tenth of new cases globally occurred in people with HIV, who are particularly susceptible to infection because HIV weakens their immune systems. Multi-drug-resistant tuberculosis is rising globally, with 161,000 cases in 2017 compared with 153,000 in 2016.
TREND WATCH
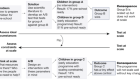
Researchers from Africa and Asia encounter much greater obstacles in getting visas to visit other countries for work than do researchers from Western nations. A global survey of academics’ movements found that Asian researchers were more than four times, and African academics more than three times, as likely as European or North American researchers to report visa-related obstacles to travelling for work. The survey polled 2,465 researchers in 109 countries, including 494 in Africa and 457 in Asia, and was conducted by the RAND Corporation for the Wellcome Trust, a research-funding charity in London, and the Together Science Can Campaign, which promotes international collaboration. The most common complaints globally involved the length of time needed to process a visa request, the complexity of application forms, costs associated with making an application and a lack of clarity on rules and procedures. Around 45% of researchers with an African nationality reported travelling to another country for work only very rarely, compared with around 17% of European researchers.