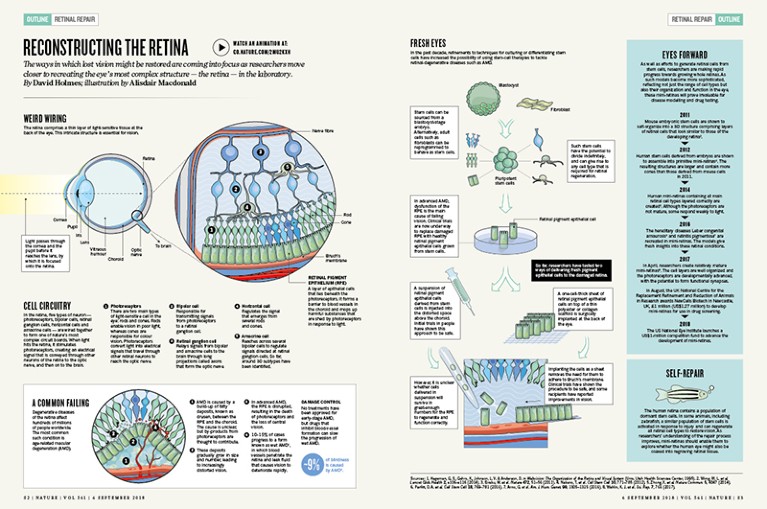
Weird wiring
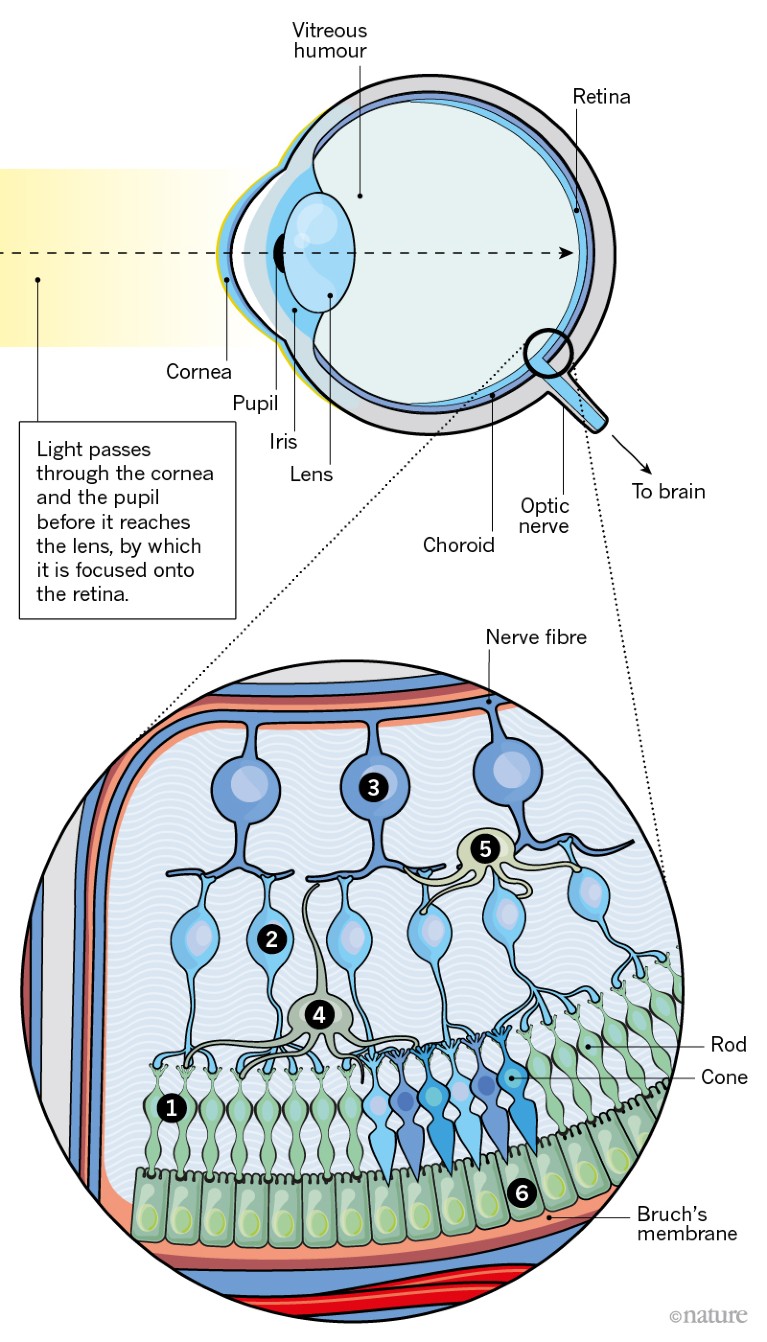
The retina comprises a thin layer of light-sensitive tissue at the back of the eye. This intricate structure is essential for vision.
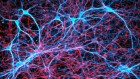
Cell circuitry
In the retina, five types of neuron — photoreceptors, bipolar cells, retinal ganglion cells, horizontal cells and amacrine cells — are wired together to form one of nature’s most complex circuit boards. When light hits the retina, it stimulates photoreceptors, creating an electrical signal that is conveyed through other neurons of the retina to the optic nerve, and then on to the brain.
Credit: Alisdair Macdonald
1. Photoreceptors There are two main types of light-sensitive cell in the eye: rods and cones. Rods enable vision in poor light, whereas cones are responsible for colour vision. Photoreceptors convert light into electrical signals that travel through other retinal neurons to reach the optic nerve.
2. Bipolar cell Responsible for transmitting signals from photoreceptors to a retinal ganglion cell.
3. Retinal ganglion cell Relays signals from bipolar and amacrine cells to the brain through long projections called axons that form the optic nerve.
4. Horizontal cell Regulates the signal that emerges from several rods and cones.
5. Amacrine cell Reaches across several bipolar cells to regulate signals directed at retinal ganglion cells. So far, around 30 subtypes have been identified.
6. Retinal pigment epithelium (RPE) A layer of epithelial cells that lies beneath the photoreceptors. It forms a barrier to blood vessels in the choroid and mops up harmful substances that are shed by photoreceptors in response to light.
A common failing
Degenerative diseases of the retina affect hundreds of millions of people worldwide. The most common such condition is age-related macular degeneration (AMD).
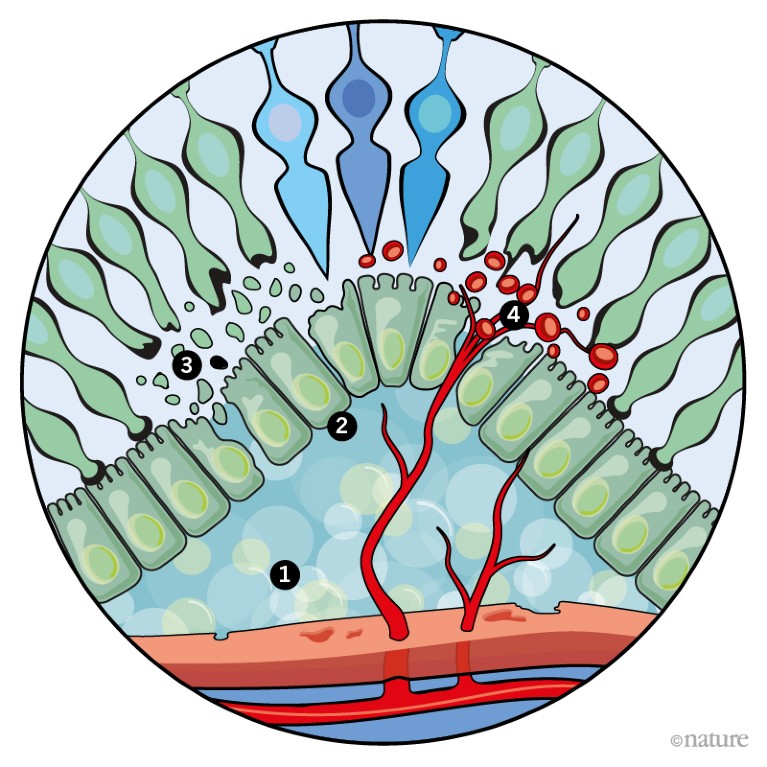
Credit: Alisdair Macdonald
1. AMD is caused by a build-up of fatty deposits, known as drusen, between the RPE and the choroid. The cause is unclear, but by-products from photoreceptors are thought to contribute.
2. These deposits gradually grow in size and number, leading to increasingly distorted vision.
3. In advanced AMD, the RPE is disrupted, resulting in the death of photoreceptors and the loss of central vision.
4. 10–15% of cases progress to a form known as wet AMD1, in which blood vessels penetrate the retina and leak fluid that causes vision to deteriorate rapidly.
Damage control
No treatments have been approved for early-stage AMD, but drugs that inhibit blood-vessel formation can slow the progression of wet AMD.
Fresh eyes
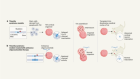
In the past decade, refinements to techniques for culturing or differentiating stem cells have increased the possibility of using stem-cell therapies to tackle retinal-degenerative diseases such as AMD.
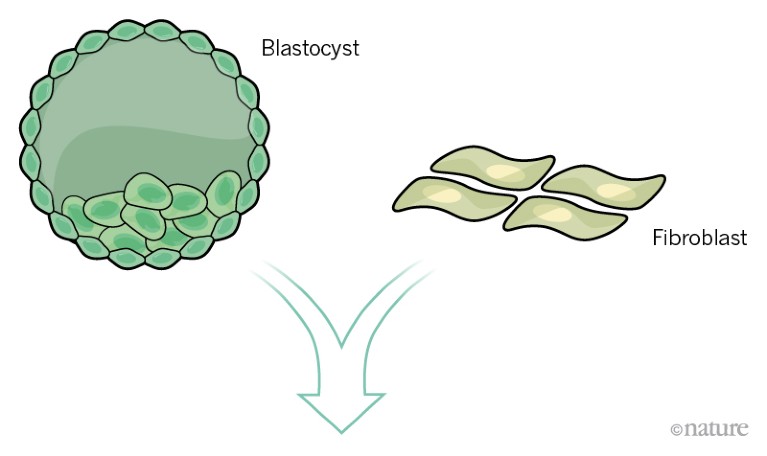
Stem cells can be sourced from a blastocyst-stage embryo. Alternatively, adult cells such as fibroblasts can be reprogrammed to behave as stem cells.
Credit: Alisdair Macdonald
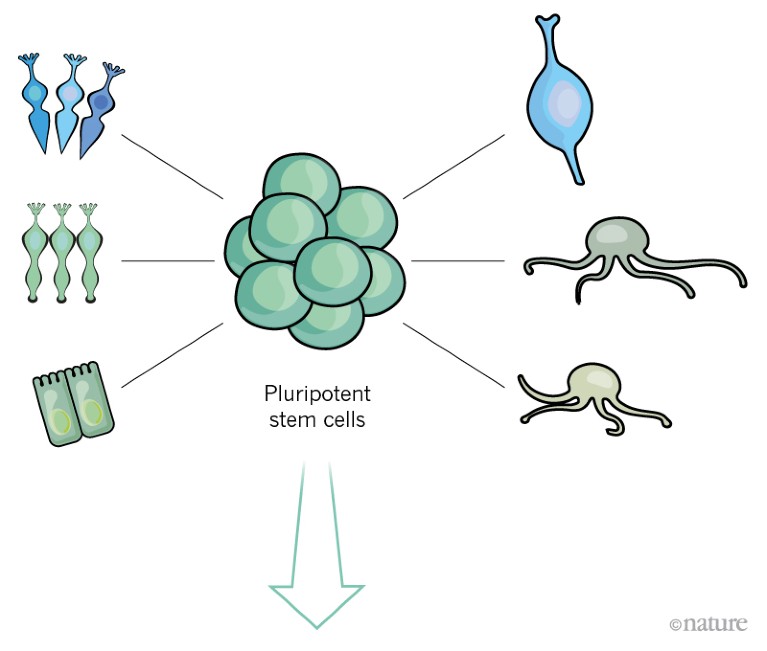
Such stem cells have the potential to divide indefinitely, and can give rise to any cell type that is required for retinal regeneration.
Credit: Alisdair Macdonald
In advanced AMD, dysfunction of the RPE is the main cause of failing vision. Clinical trials are now under way to replace damaged RPE with healthy retinal pigment epithelial cells grown from stem cells.
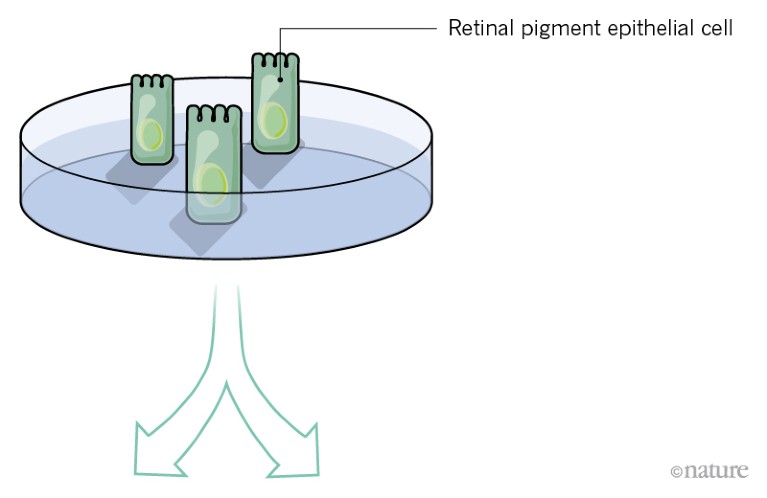
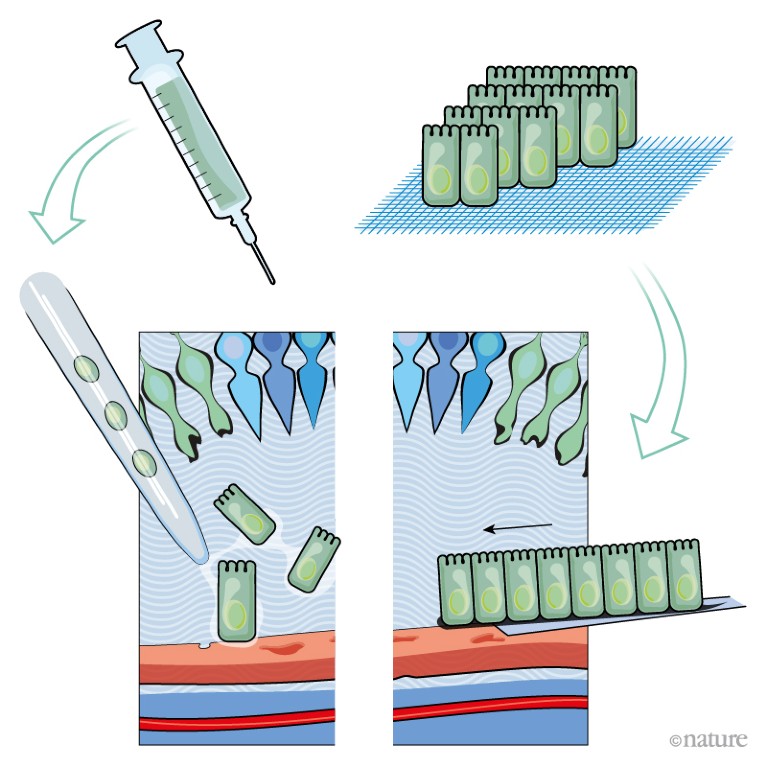
So far, researchers have tested two ways of delivering fresh pigment epithelial cells to the damaged retina.
Credit: Alisdair Macdonald
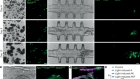
A, A suspension of retinal pigment epithelial cells derived from stem cells is injected into the distorted space above the choroid. Initial trials in people have shown this approach to be safe.
However, it is unclear whether cells delivered in suspension will survive in great-enough numbers for the RPE to regenerate and function correctly.
B, A one-cell-thick sheet of retinal pigment epithelial cells on top of a thin polyester or collagen scaffold is surgically implanted at the back of the eye.
Implanting the cells as a sheet removes the need for them to adhere to Bruch’s membrane. Clinical trials have shown the procedure to be safe, and some recipients have reported improvements in vision.
Credit: Alisdair Macdonald
Self-repair
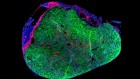
The human retina contains a population of dormant stem cells. In some animals, including zebrafish, a similar population of stem cells is activated in response to injury and can regenerate all retinal cell types to restore vision. As researchers’ understanding of the repair process improves, mini-retinas should enable them to explore whether the human eye might also be coaxed into regrowing retinal tissue.
Credit: Alisdair Macdonald

 Nature Outline: Retinal repair
Nature Outline: Retinal repair
 Retinal repair: visions of the future
Retinal repair: visions of the future