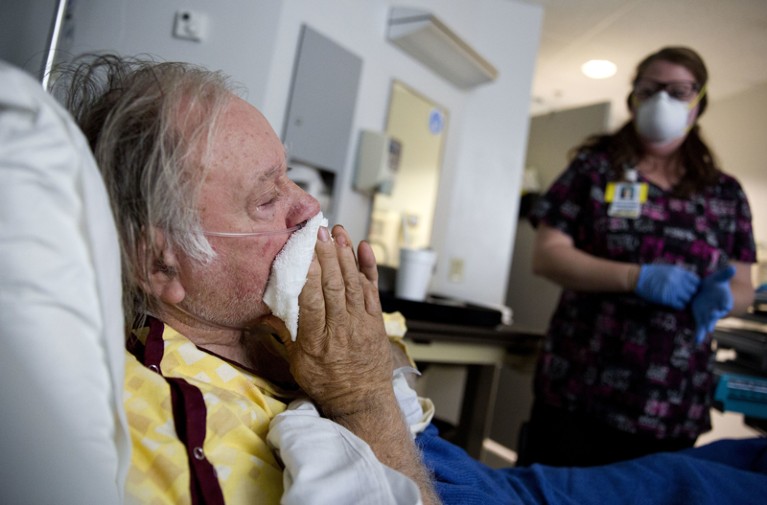
Basic and applied science are at last offering hope against influenza.Credit: David Goldman/AP/REX/Shutterstock
Hundreds of thousands of people die every year from influenza or a related condition. Unlike chicken pox and many other viruses in which the initial infection often provides lifelong immunity, the flu virus is constantly evolving. As a result, although most children will have been infected by the time they are around 3 years old, they will encounter new bouts of flu every 5–10 years.
As we highlight in a News Feature this week, there is increasing scientific interest in how the human immune system is primed by its first exposure to flu in childhood. This immunologic ‘imprinting’ in part explains the vastly divergent susceptibilities of people born in different years to seasonal outbreaks of flu. The closer the characteristics of the circulating virus to the strain a person first experienced, the stronger their natural defences against it.
The ghost of influenza past and the hunt for a universal vaccine
Such insights could help researchers to design more-effective vaccines. The need is great. In a good year, seasonal vaccines might protect six in ten people from infection, and this protection begins to wane after a few months. By contrast, a single shot of a vaccine against yellow fever is more than 99% effective and confers lifelong protection.
In the 2017–18 flu season, a virus subtype called H3N2 has predominated in many countries. In those places, the efficacy of the available vaccine has been much worse: just 10% in Australia, 17% in Canada and 25% in the United States. That’s better than nothing, and is surely saving many precious lives — especially young children, who are particularly vulnerable. But it’s hardly optimal.
The US Centers for Disease Control and Prevention notes that 80% of the children who died from flu this year in the United States had not been vaccinated. At present, many adults don’t get immunized. A more-effective, longer-lasting vaccine would increase uptake, and would also probably reach the threshold to achieve collective — herd — immunity, thus reducing the number of people who could pass on the infection.
This logic is all the more important when it comes to the many lower- and middle-income countries that have few, if any, flu-vaccination programmes, simply because the costs and logistics of re-formulating and re-administering vaccines every year are prohibitive. Less-onerous vaccine requirements would encourage these countries to vaccinate their populations — and help to reduce the 290,000–650,000 deaths estimated by the World Health Organization to occur worldwide every year from flu-related respiratory diseases. Better still would be universal flu vaccines that would confer immunity against all new flu subtypes that emerge to cause pandemics.
Many scientists are increasingly confident that such a breakthrough is in reach. Are they right? Research over the past decade suggests that developing such vaccines is doable. This work includes findings that childhood imprints ‘memorize’ regions of the virus that mutate little and so differ little between flu subtypes. This memory is dormant, but it is there; a vaccine that could ‘wake up’ this memory should therefore produce broadly reactive antibodies that protect against multiple flu strains and subtypes. Technological advances, such as single-cell sorting and sequencing, are revolutionizing scientists’ ability to characterize in great depth the function of cell types involved in the host immune response.
Much essential information is expected to flow from a new large cohort study funded by the US National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland. Starting next year, it will monitor newborns from several countries over multiple flu seasons to see how their first flu infections, and any subsequent ones (and vaccinations), affect their immune systems. It will also chart how they respond to new exposures, and so help to unravel the mysteries of the mechanisms of their immunologic imprinting.
Commendably, the NIAID has stipulated that all data and clinical samples be shared widely with other researchers. This is important, because not only are such large and intensive cohort studies expensive and hard to run, but this study is of infants — meaning it’s crucial to minimize blood draws and make the most of each sample.
We still have a way to go. But protective and longer-lasting vaccines seem to be well on their way.

 The ghost of influenza past and the hunt for a universal vaccine
The ghost of influenza past and the hunt for a universal vaccine
 Science must get ready for the next global flu crisis
Science must get ready for the next global flu crisis
 Mutations explain poor showing of 2012 flu vaccine
Mutations explain poor showing of 2012 flu vaccine