Credit: Univ. Minnesota Archives
Paul Boyer was approaching the finish line of his career when he risked everything with a jaw-dropping proposal. He addressed one of the most important, as-then-unanswered questions in biochemistry: how do cells use an electrochemical gradient to form the molecule adenosine triphosphate (ATP), which drives the energy-requiring processes that are essential to life?
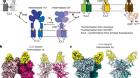
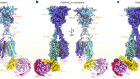
More than 90% of the ATP formed in our cells is catalysed by an enzyme called ATP synthase. Boyer’s remarkable proposal for how the enzyme works was that ATP synthase functions as a tiny molecular motor. Just as electric motors spin when electrons flow through a potential gradient, a flow of protons (hydrogen ions) across an electrochemical gradient generated by respiration causes the core of the ATP synthase to spin relative to its surrounding catalytic subunits. Because the core is asymmetric, its rotation causes the surrounding subunits to change their shape, thus disrupting the tight binding site where ATP is formed, allowing its release.
Boyer died on 2 June, eight weeks shy of his 100th birthday. Born in Provo, Utah, he studied chemistry at Brigham Young University, also in Provo. After marrying his college sweetheart, Lyda Whicker, he headed in 1939 to the biochemistry department at the University of Wisconsin–Madison for his PhD degree. After postdoctoral training at Stanford University, California, in 1946 he accepted a faculty position at the University of Minnesota, St Paul. In 1963 he returned to California to head the biochemistry division of the chemistry department at the University of California, Los Angeles (UCLA), where he spent the rest of his career.
Boyer’s revelations about ATP synthase occurred in three steps and involved insights that defied dogma. I was fortunate to be a postdoc in Paul’s lab when he came up with the first of these.
We were attending a UCLA seminar in 1972 when I noticed that he wasn’t paying attention to the speaker. Afterwards, Paul approached us in a very excited state. This was surprising because he was known for his calm demeanour. He confessed that he had spent the hour thinking about old unexplained data. He asked: “What would you say if I told you that it doesn’t take energy to make ATP at the catalytic site of ATP synthase,” (as was universally held at the time) “but rather that it takes energy to get ATP off the catalytic site?” This was a eureka moment.
In other words, tightly bound ATP forms spontaneously at catalytic sites on the synthase. Energy from the electrochemical gradient is used to alter the surrounding structure, which disrupts the tight site, allowing ATP release.
As is often the case with transformational ideas, early reactions were negative. When the Journal of Biological Chemistry rejected our manuscript containing data supporting this concept, Boyer told me without animosity that he could see why they would do that — “It was a very striking claim.” The work was published in 1973 in the Proceedings of the National Academies of Science.
A second feature of the mechanism, on which he published a 1977 paper, involved recognizing that the multiple catalytic sites of the synthase coordinated with one another: the finished ATP product is released only when ATP precursors bind at another, adjacent site.
Finally, in 1981–82, Boyer proposed that rotation of the asymmetric core of the synthase, driven by an electrochemical gradient, led to the necessary changes in the surrounding catalytic subunits.
Subsequently, all three concepts received strong experimental support from numerous labs. This included results of X-ray crystallographic studies conducted by John Walker at the UK Medical Research Council’s Laboratory of Molecular Biology in Cambridge; he was also a recipient of the 1997 Nobel Prize in Chemistry.
Boyer had a gift for extracting insights from data. But he also drew on knowledge acquired over years of reading beyond his field. As editor of 19 volumes in a series of books called The Enzymes, and as co-editor and editor of the Annual Review of Biochemistry, he followed all the major advances in enzymology over several decades. He also gained an edge by coming to biochemistry as a chemist, not as a biologist as was common in the 1940s. His firm understanding of kinetics and thermodynamics played an important part in his discoveries.
Boyer was a highly effective trainer of young scientists. Mentoring by example, he exhibited an incredible work ethic, a love for science and a pure joy in discovery. He referred to his favourite enzymes as friends. He set high expectations while providing a supportive and stimulating environment. Even after you left his lab, he looked for opportunities to help you advance your career.
Paul was also a role model for how to live your life with integrity, respect for others and kindness. He showed exceptional civility in a field known to be contentious (shouting matches were common at scientific meetings). In all the years we worked together, I never heard him raise his voice in anger or offer a personal criticism of any of his competitors. When faced with a difficult situation, I still ask myself: how would Paul Boyer handle this problem? The answer is always: with grace and generosity.

 Experimentalists and theorists need to talk
Experimentalists and theorists need to talk
 The next big hit in molecule Hollywood
The next big hit in molecule Hollywood