Illustration by Chris Gash
Matt Trusheim flips a switch in the darkened laboratory, and an intense green laser illuminates a tiny diamond locked in place beneath a microscope objective. On a computer screen an image appears, a fuzzy green cloud studded with brighter green dots. The glowing dots are color centers in the diamond—tiny defects where two carbon atoms have been replaced by a single atom of tin, shifting the light passing through from one shade of green to another.
Later, that diamond will be chilled to the temperature of liquid helium. By controlling the crystal structure of the diamond on an atom-by-atom level, bringing it to within a few degrees of absolute zero and applying a magnetic field, researchers at the Quantum Photonics Laboratory run by physicist Dirk Englund at the Massachusetts Institute of Technology think they can select the quantum-mechanical properties of photons and electrons with such precision that they can transmit unbreakable secret codes.
Trusheim, a postdoctoral researcher in the lab, is one of many scientists trying to figure out just which atoms embedded in which crystals under what conditions will give them that kind of control. Indeed, scientists around the world are tackling the hard problem of controlling nature at the level of atoms and below, down to electrons or even fractions of electrons. Their aim is to find the knobs that control the fundamental properties of matter and energy and turn those knobs to customize matter and energy, creating ultrapowerful quantum computers or superconductors that work at room temperature.
Innovations In The Biggest Questions In Science
These scientists face two main challenges. On a technical level, the work is extremely difficult. Some crystals, for instance, must be made to 99.99999999 percent purity in vacuum chambers emptier than space. The more fundamental challenge is that the quantum effects these researchers want to harness—for example, the ability of a particle to be in two states at once, à la Schrödinger’s cat—happen at the level of individual electrons. Up here in the macro world, the magic goes away. Researchers manipulating matter at the smallest scales, therefore, are trying to coax nature into behaving in ways that strain at the limits imposed by fundamental physics. The degree to which they succeed will help determine our scientific understanding and technological capacity in the decades to come.
An Alchemist’s Dream
Manipulating matter is, to a significant degree, all about controlling electrons. After all, the behavior of the electrons in a material determines its properties as a whole—whether the substance is a metal, an insulator, a magnet, or something else. Some scientists are attempting to alter the collective behavior of electrons to create what is known as quantum synthetic materials. Researchers envision that “we can take an insulator and make it into a metal or a semiconductor and make it into a superconductor. We can turn a nonmagnetic material into a magnetic material,” asserted physicist Eva Andrei of Rutgers University at a recent conference. “This is really an alchemist’s dream come true.”
The dream could lead to actual breakthroughs. For example, researchers have been trying for decades to create room-temperature superconductors, materials that could yield innovations such as electrical transmission lines that do not lose any energy. In a breakthrough in 1957 that earned them a Nobel Prize in 1972, physicists John Bardeen, Leon Cooper and John Robert Schrieffer demonstrated that superconductivity arises when the free electrons in a metal such as aluminum align into so-called Cooper pairs. Even if they are relatively far apart, each electron is matched with another that has the opposite spin and momentum. Rather like couples dancing in a crowded disco, the paired electrons’ motions are coordinated with each other even if other electrons come between them.
This arrangement allows current to flow through a material with no resistance and therefore no loss. The only practical superconductors developed thus far must be cooled to within a few degrees of absolute zero before this state takes hold. Yet recently researchers have found that hitting a material with a high-intensity laser can also knock electrons into Cooper pairs, if only briefly. David Hsieh, a condensed matter physicist at the California Institute of Technology, creates photoinduced superconductivity in a type of material (known as a Mott insulator) that becomes insulating at very cold temperatures. Light striking the insulator excites the electrons, causing them to briefly align. “The shaking needs to be done very violently,” Hsieh explains. “Momentarily, the electric field is extremely strong—but it’s only on for such a short time [that] it’s not delivering that much heat.”
To keep the laser from vaporizing the material, Hsieh strikes it with a pulse that lasts only tens or hundreds of femtoseconds. (There are as many femtoseconds in one second as there are seconds in 32 million years.) Unfortunately, the superconductivity thereby induced does not last much longer. The challenge for researchers pursuing similar work is to figure out how to make the effect last long enough to be useful. Hsieh says of this and other studies of quantum materials: “What we’re trying to do is dream up host compounds in which even when you’re talking about a large batch of electrons, that quantum-mechanical weirdness that typically is confined to single particles is still retained.”
Unbreakable Codes
Controlling electrons is also how Trusheim and Englund hope to develop unbreakable quantum encryption. In their case, the goal is not to change the properties of materials but to share the quantum properties of electrons within their engineered diamonds with photons that transmit the cryptographic key. Rattling around in the color centers of the diamonds in Englund’s lab are free electrons, the spins of which can be measured by probing them with a strong magnetic field. A spin that is aligned with the field can be called spin 1, and a spin that is not aligned is spin 2—equivalent to the 1 and 0 of a digital bit. “It’s a quantum particle, so it can be in both states at the same time,” Englund says. That makes it a quantum bit, or qubit, capable of making multiple calculations simultaneously.
This is where a mysterious property known as quantum entanglement comes in. Imagine a box containing a red ball and a blue ball. You can reach in without looking, take one ball and put it in your pocket, then travel across town. You then take the ball out of your pocket and discover that it is red. That immediately tells you that the ball back in the box is blue. That is entanglement. This effect, translated to a quantum realm, can transmit information instantaneously and across vast distances.
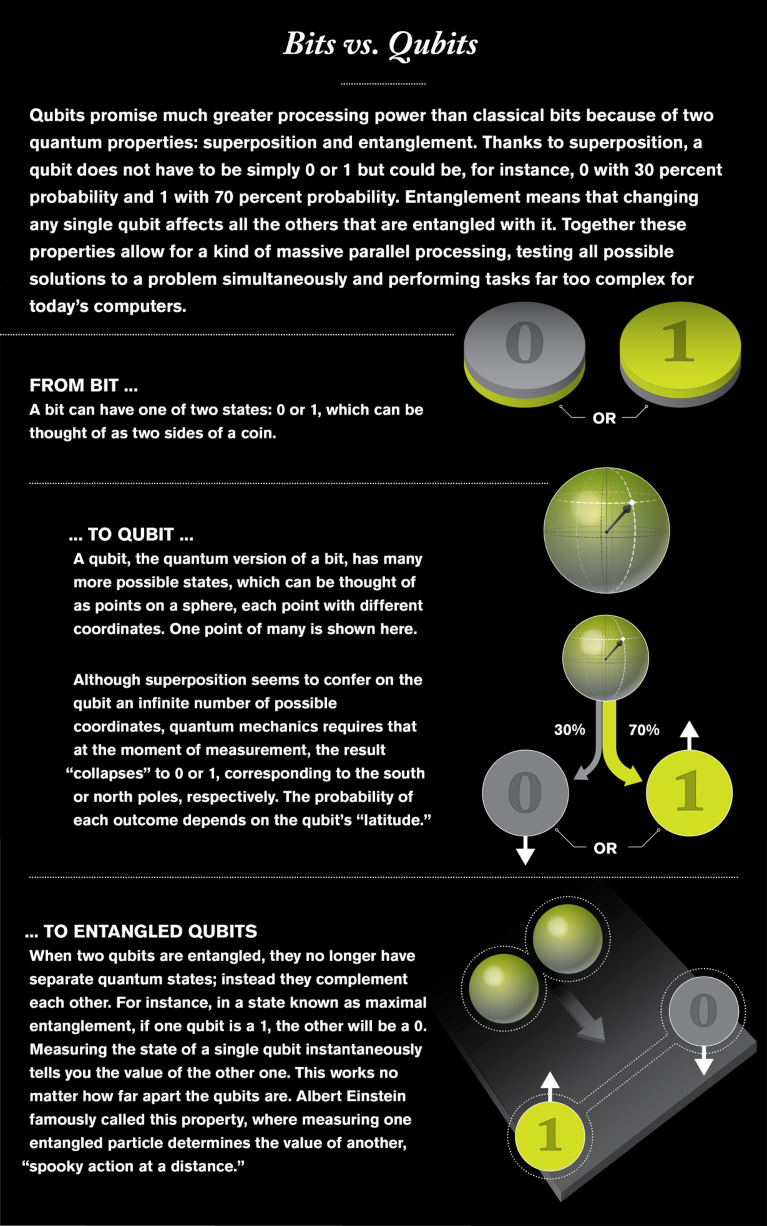
Illustration by Jen Christiansen
The color centers in the diamonds in Englund’s lab transfer the quantum states of the electrons contained within to photons by means of entanglement, creating what Englund calls “flying qubits.” As in standard optical communications, a photon can be transmitted to a receiver—in this case, another diamond vacancy—and its quantum state transferred to a new electron, so the two electrons become correlated. The transmission of such entangled bits allows two people to share a cryptographic key. “Each one has a string of 0s and 1s, or ups and downs of the spin, that look locally random, but they’re identical,” Englund says. Using that key as a multiplication factor for other data they send lets them communicate securely. If an eavesdropper were to intercept the transmission, the senders would know because the act of measuring a quantum state changes it.
Englund is experimenting with a quantum network that sends photons over optical fibers between his lab, a facility down the road at Harvard University, and another at M.I.T.’s Lincoln Laboratory in the nearby town of Lexington, Mass. Researchers have already succeeded in transmitting quantum-cryptographic keys over greater distances—in 2017 Chinese scientists reported having transmitted such a key from a satellite in Earth orbit to two ground stations 1,200 kilometers apart in the mountains of Tibet. But the bit rate of the Chinese experiment was too low for practical communications: the researchers detected only one entangled pair out of six million. The innovation that will make ground-based quantum-cryptographic networks practical are quantum repeaters—devices placed at intervals throughout the network that boost the signal without interfering with its quantum properties. Englund’s goal is to find materials with just the right atomic defects to form the heart of those quantum repeaters.
The trick is making enough spin-entangled photons to carry the data. The electron in a nitrogen vacancy maintains its spin state for a long time—about a second—increasing the number of chances for laser light passing through to produce an entangled photon. But nitrogen is a small atom, and it does not fill the space created by the missing carbons. This misfit can cause subsequent photons to be of slightly different colors, so they no longer match one another. Other atoms, such as tin, nestle snugly and produce a stable wavelength. But those atoms do not hold their spin as long—hence the work continues to find the perfect balance.
Split Ends
While Englund and others wrestle with individual electrons, some scientists are diving even deeper into the quantum world and trying to manipulate mere fractions of electrons. This work has its roots in an experiment conducted in 1982, when scientists from Bell Laboratories and Lawrence Livermore National Laboratory sandwiched two layers of different semiconductor crystals together, cooled them to near absolute zero and applied a strong magnetic field, trapping electrons in a plane at the interface between the two crystal layers. This arrangement created a kind of quantum soup, in which the movement of any given electron is influenced by the charges it feels from other electrons. “They’re not really individual particles per se,” says Michael Manfra, who runs the Quantum Semiconductor Systems Group at Purdue University. “You can imagine a ballet where each dancer is not just doing their own thing, but they’re responding to the motion of either their partner or the other dancers. There’s this sort of generalized response.”
The odd thing about this collection is that it can have fractional charges. An electron is an indivisible unit—you cannot slice one into thirds—but a group of electrons in the right state can produce a so-called quasiparticle with a 1/3 charge. “It’s as if electrons are fractionalized,” says Mohammad Hafezi, a physicist at the Joint Quantum Institute, a research partnership between the University of Maryland and the National Institute of Standards and Technology. “It is very strange.” Hafezi creates the effect in supercooled graphene, one-atom thick sheets of carbon, and he recently showed he could manipulate the movement of the quasiparticles by shining a laser on the graphene. “Now it’s controllable,” he says. “Now the external knobs I have, like the magnetic field and the light, can be tuned up and down. So the nature of that collective state changes.”
Manipulating quasiparticles could allow for the creation of a special kind of qubit—a topological qubit. Topology is a field of mathematics that studies properties of an object that do not change even when that object is twisted or deformed. The standard example is a doughnut: if it were perfectly elastic, you could reshape it into a coffee cup without changing anything essential; the doughnut hole would take on a new role as the opening in the cup’s handle. To change the doughnut to a pretzel, however, you would have to poke new holes into it, changing its topology.
A topological qubit retains its properties even under changing conditions. Normally particles change their quantum states, or “decohere,” when disturbed by something in their environment, such as a tiny vibration caused by heat. But if you make a qubit from two quasiparticles separated by some distance—say at opposite ends of a nanowire—you are essentially splitting an electron. Both “halves” would have to experience the exact same disturbance to decohere, and that is unlikely to happen by chance.
That property makes topological qubits attractive for quantum computers. Because of the ability of a qubit to be in a superposition of many states at once, quantum computers should be able to perform otherwise impossibly calculation-intensive tasks such as modeling the physics of the big bang. Manfra, in fact, is part of Microsoft’s global effort to build quantum computers based on topological qubits. There are other, arguably easier approaches. Google and IBM, for example, are pursuing quantum computers based on wires supercooled to become semiconductors or ionized atoms in a vacuum chamber trapped by lasers. The problem with those approaches is that they are more sensitive to environmental perturbations than topological qubits, especially as the number of qubits grows.
Topological qubits could therefore herald a revolution in our ability to manipulate tiny things. There is, however, one significant problem: they do not yet exist. Researchers are struggling to construct them out of an object called a Majorana particle. Hypothesized by Ettore Majorana in 1937, this particle is its own antiparticle. An electron and its antiparticle, a positron, have identical properties except for charge, but the charge of the Majorana particle would be zero.
Scientists believe that certain configurations of electrons and holes (absences of electrons) can behave like Majorana particles. These, in turn, may one day be used as topological qubits. In 2012 physicist Leo Kouwenhoven of Delft University of Technology in the Netherlands and his colleagues measured what seemed to be Majorana particles in a network of superconducting and semiconducting nanowires. Still, argues Sankar Das Sarma of the Condensed Matter Theory Center at the University of Maryland, College Park, the only way to actually prove that these quasiparticles exist would be to build a topological qubit out of them.
Other experts in the field are optimistic, however. “I think without any question, eventually somebody will make a topological qubit, just because it’s interesting to do, and they’ll figure out how to do it,” says Steve Simon, a condensed matter theorist at the University of Oxford. “The big question is, Is this the way we’re going to build a quantum computer in the future?”
Quantum computers—along with high-temperature superconductors and unbreakable quantum encryption—may be years away, or they may never be achieved. But in the meantime, researchers will continue to struggle toward mastery of nature at the smallest scales. Scientists do not yet know how low they can go. They have gone surprisingly far, but the further down they get, the more nature pushes back.

 Looking at the Stars
Looking at the Stars
 What Is Spacetime?
What Is Spacetime?
 What Is Dark Matter?
What Is Dark Matter?
 What Is Consciousness?
What Is Consciousness?
 How Did Life Begin?
How Did Life Begin?
 How Much Can We Know?
How Much Can We Know?






