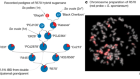
- NEWS AND VIEWS
Peptide signal alerts plants to drought
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 556, 178-179 (2018)
doi: https://doi.org/10.1038/d41586-018-03872-4
References
Zhu, J.-K. Cell 167, 313–324 (2016).
Takahashi, F. et al. Nature 556, 235–238 (2018).
Nambara, E. & Marion-Poll, A. Annu. Rev. Plant Biol. 56, 165–185 (2005).
Kim, T.-H., Böhmer, M., Hu, H., Nishimura, N. & Schroeder, J. I. Annu. Rev. Plant Biol. 61, 561–591 (2010).
Matsubayashi, Y. Annu. Rev. Plant Biol. 65, 385–413 (2014).
Ito, Y. et al. Science 313, 842–845 (2006).
Endo, A. et al. Plant Physiol. 147, 1984–1993 (2008).
Lenhard, M. & Laux, T. Development 130, 3163–3173 (2003).
Christmann, A., Grill, E. & Huang, J. Curr. Opin. Plant Biol. 16, 293–300 (2013).
DeYoung, B. J. et al. Plant J. 45, 1–16 (2006).
Yang, Z. et al. Proc. Natl Acad. Sci. USA 113, 6791–6796 (2016)

 Read the paper: A small peptide modulates stomatal control via abscisic acid in long-distance signalling
Read the paper: A small peptide modulates stomatal control via abscisic acid in long-distance signalling
 A cellular passage to the root interior
A cellular passage to the root interior
 Forests on the brink
Forests on the brink